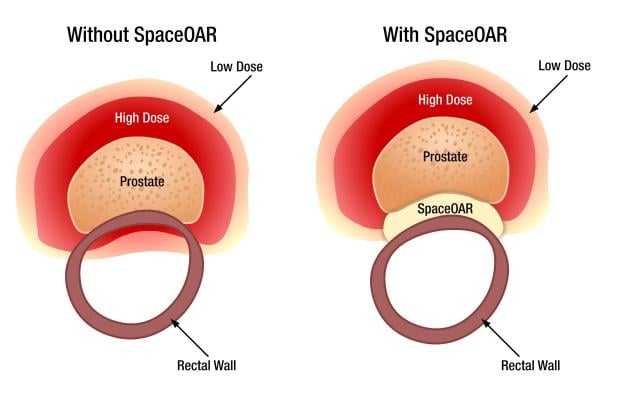
April 6, 2015 — Augmenix Inc. announced U.S. Food and Drug Administration (FDA) clearance of the SpaceOAR System, cleared as a spacer to protect the rectum in men undergoing prostate cancer (PCa) radiotherapy. Despite many advancements in PCa radiotherapy, the close prostate-rectum proximity makes it challenging to deliver adequate radiation to the prostate without injuring the rectum (defined as the Organ At Risk - OAR). The SpaceOAR System is intended to temporarily position the anterior rectal wall away from the prostate during radiotherapy for PCa.
Placed through a small needle, the hydrogel is administered as a liquid, but quickly solidifies into a soft gel that expands the space between the prostate and rectum. The hydrogel spacer maintains this space until radiotherapy is complete. The spacer then liquefies and is absorbed and cleared from the body in the patient’s urine.
“SpaceOAR hydrogel is a valuable new tool for urologists and radiation oncologists and should be instrumental in the adoption of advanced prostate cancer radiotherapy protocols,” said John Sylvester, M.D., of Lakewood Ranch Oncology in Bradenton, Florida, and a principal investigator in the SpaceOAR clinical trial. “Shielding the rectum from radiation may enable the utilization of dose escalation (more prostate radiation for improved cancer kill rates) and hypofractionation (fewer radiation treatment sessions), both of which should have substantial patient benefits and help reduce healthcare costs.”
According to the American Cancer Society and the National Cancer Institute, prostate cancer is second only to skin cancer as the most frequently diagnosed cancer in men with an estimated 220,800 new cases and 27,540 deaths in the United States in 2015 alone. Worldwide PCa is expected to grow to 1.7 million new cases and 499,000 deaths by 2030 due to the aging population. Following PCa diagnosis, treatment options are usually limited to surgery, radiotherapy or active surveillance, where no immediate therapeutic treatment occurs.
FDA clearance was granted following completion of the SpaceOAR System prospective, multicenter, randomized clinical trial. SpaceOAR patients experienced a significant reduction in rectal radiation dose and severity of late rectal toxicity when compared to control patients who did not receive SpaceOAR hydrogel. The full pivotal clinical trial results have been submitted for publication in a peer-reviewed journal and are expected to be published later this year.
The SpaceOAR System is also CE marked and TGA approved.
For more information: www.augmenix.com


 January 30, 2026
January 30, 2026 









