March 22, 2013 — GE Healthcare announced the U.S. Food and Drug Administration (FDA) approval of a new indication for AdreView (Iobenguane I 123 Injection), the first and only FDA approved molecular imaging agent to link nerve function in the heart to a patient’s mortality risk.[1] AdreView is approved for the scintigraphic assessment of myocardial sympathetic innervation (cardiac nerve activity) to assist in the evaluation of patients with New York Heart Association (NYHA) Class II or Class III heart failure and left ventricular ejection fraction (LVEF) ? 35 percent.[2]
“Predicting disease progression in heart failure patients can be difficult, and there are currently a limited number of prognostic tools available to help clinicians understand the likelihood for heart failure progression,” said James Arrighi, M.D., associate professor of medicine, Brown University, Providence, R.I., and current president of the American Society of Nuclear Cardiology. “Now, with AdreView, we have a tool that will provide clinicians with a numeric score to help stratify mortality risk, and may help to promote more informed clinical decision-making.”
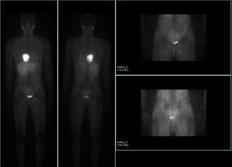
Increased myocardial sympathetic activity is a prominent feature of heart failure[3] and is often associated with decline in left ventricular function, worsening heart failure symptoms and sudden cardiac death.[1,4] This increase leads to a depletion of norepinephrine (NE) storage and uptake.[5] AdreView provides a means for assessing the neuronal capacity for uptake and storage of NE.[2] While current prognostic tests look at the effect of the disease on heart muscle and blood flow, imaging with AdreView uses the heart to mediastinum (H/M) ratio to assess the functionality of the sympathetic nerves.
With AdreView, the H/M ratio is a measure of radioactivity uptake in the heart compared to that of a reference region in the mediastinum (the mass of tissues and organs between the two pleural sacs that separate the heart from the lungs).[2,6] This measurement has a typical range of 1-2.4 and can accurately identify patients with lower than average one- and two- year mortality risk. In clinical studies, an AdreView Score (H/M ratio) of ?1.6 was associated with a 99 percent probability of survival at one year (negative predictive value, NPV).[2] In patients with congestive heart failure, AdreView utility has not been established for selecting therapy, monitoring response to therapy, or to identify a patient with a high risk for death.[2]
“GE Healthcare is committed to providing innovative and effective medical products that will aid physicians in determining mortality risk in patients with cardiovascular disease, and we are pleased that the FDA has approved AdreView for this indication in heart failure patients,” said Terri Moench, general manager, SPECT, GE Healthcare medical diagnostics. “The use of imaging tests is consistent with current trends towards gaining improved and earlier understanding of heart disease at a molecular level and may enable providers to help deliver better care to more people.”
The safety and efficacy of AdreView were evaluated in two open-label, multicenter, international trials in 985 patients with NYHA class II or class III heart failure with LVEF ?35 percent. A total of 110 patients without a history of heart disease also received a single dose of AdreView. The endpoint for analysis was all-cause mortality, an outcome confirmed by review of an independent adjudication committee. By 12 months following enrollment among the 964 heart failure patients in the efficacy population, 50 (5%) patients had died, 61 (6%) had missing follow-up information and three patients had missing H/M ratios. One-year mortality rates in relation to AdreView H/M ratio were: <1.2: 13.4%; 1.2-1.6: 5.5%; ?1.6: 1.0%. By 23 months following enrollment (the requirement for designation of two-year follow-up), 96 (10%) patients had died, 201 (21%) patients had missing follow-up information and three patients were missing H/M ratio data. Two-year mortality rates in relation to AdreView H/M ratio were: <1.2: 22.0%; 1.2-1.6: 11.5%; ?1.6: 3.3%.[2]
For more information: newsroom.gehealthcare.com
References:
1. Jacobson AF, Senior R, Cerqueira MD, et al. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure: results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) Study. J Am Coll Cardiol. 2010;55:2212-2221
2. AdreView Prescribing Information, GE Healthcare. 2013.
3. Leimbach WN Jr., Wallin BG, Victor RG, Aylward PE, Sundlof G, Mark AL. "Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure." Circulation 1986;73:913–9.
4. Brunner-La Rocca HP, Esler MD, Jennings GL, Kay DM. "Effect of cardiac nervous activity on mode of death in congestive heart failure." Eur Heart J 2001;22:1136–43.
5. Eisenhafer G, Friberg P, Rundqvist B, et al. "Cardiac sympathetic nerve function in congestive heart failure." Circulation. 1996;93:1667-1676.
6. The American Heritage Medical Dictionary. http://medical-dictionary.thefreedictionary.com/mediastinum. Accessed March 2013.
7. Hall MJ, Levant S, DeFrances C. "Hospitalization for Congestive Heart Failure: United States, 2000-2010." U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. 2012.
8. Go AS, Mozaffarian D, Roger VL, et al. "Heart disease and stroke statistics — 2013 update: A report from the American Heart Association." Circulation.2013;127:e6-e245.
9. National Heart, Lung, and Blood Institute. "Congestive Heart Failure: A New Epidemic in the United States." Data Fact Sheet. September 1996


 February 03, 2026
February 03, 2026 









