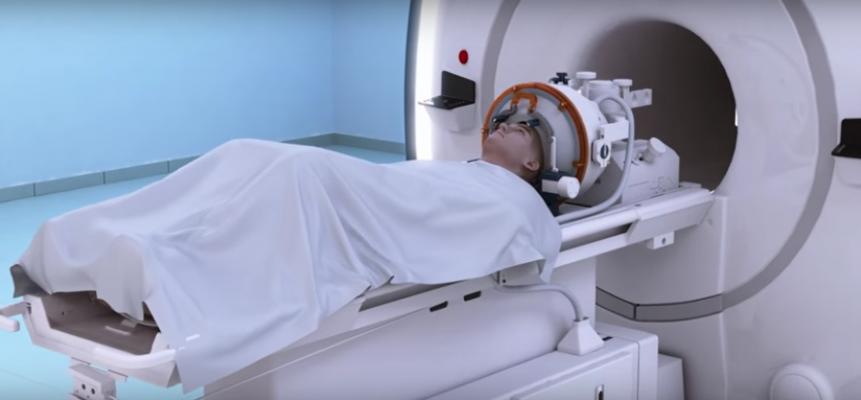
July 11, 2016 — The U.S. Food and Drug Administration (FDA) recently approved the first focused ultrasound device to treat essential tremor in patients who have not responded to medication. ExAblate Neuro uses magnetic resonance (MR) images taken during the procedure to deliver focused ultrasound to destroy brain tissue in a tiny area thought to be responsible for causing tremors.
“Patients with essential tremor who have not seen improvement with medication now have a new treatment option that could help them to avoid more invasive surgical treatments,” said Carlos Peña, Ph.D., M.S., director of the division of neurological and physical medicine devices in the FDA’s Center for Devices and Radiological Health. “As with other treatments for essential tremor, this new device is not a cure but could help patients enjoy a better quality of life.”
Essential tremor, also called benign essential tremor, is the most common form of tremor. According to the National Institute of Neurological Disorders and Stroke, several million Americans, usually those over age 40, are affected by the condition. Essential tremor may be treated with beta blockers or anticonvulsant drugs. If medications fail to control symptoms, the condition may also be treated with surgery (thalamotomy) or a deep brain stimulation device to destroy the tiny part of the brain (thalamus) that controls some involuntary movements.
To determine if the ExAblate Neuro treatment is appropriate, patients should first have MR and computerized tomography (CT) scans. Those undergoing treatment with the MRI-guided device lie in an MRI scanner that takes images to help a doctor identify the targeted area in the brain’s thalamus for treatment. Treatment with transcranial focused ultrasound energy is administered with incremental increases in energy until patients achieve a reduction of tremor. Patients are awake and responsive during the entire treatment.
Data supporting the safety and effectiveness of the device system included a double-blind control trial involving 76 patients with essential tremor who had not responded to medication therapy. Fifty-six of the patients were randomly selected to receive the ExAblate Neuro treatment and 20 received a fake treatment. Patients in the control group were able to cross over into the treatment group three months later.
Patients treated with the ExAblate Neuro showed nearly a 50 percent improvement in their tremors and motor function (composite tremor/motor function score) three months after treatment compared to their baseline score. Patients in the control group had no improvement, and some experienced a slight worsening after the sham procedure before they crossed over into the treatment group. At 12 months post-procedure, the treatment group retained a 40 percent improvement in these scores compared to baseline.
Adverse events for the ExAblate Neuro are consistent with those reported for thalamotomy surgery, including numbness/tingling of the fingers, headache, imbalance/unsteadiness, loss of control of body movements (ataxia) or gait disturbance. Other side effects identified as possibly related to treatment with MR-guided focused ultrasound treatments include tissue damage in an area other than the treatment area, hemorrhage in the treated area requiring emergency treatment, skin burns with ulceration of the skin, skin retraction and scar formation and blood clots.
The ExAblate Neuro treatment is contraindicated for patients who cannot have MRI, including those who have a non-MRI compatible implanted metallic device such as a cardiac pacemaker, those with allergies to MR contrast agents or those with body size limitations for MR.
The treatment should also not be used in women who are pregnant, patients with advanced kidney disease or on dialysis, those with unstable heart conditions or severe hypertension, patients exhibiting any behavior consistent with ethanol or substance abuse or patients with a history of abnormal bleeding, hemorrhage and/or blood clotting disorders (coagulopathy). Patients currently taking anticoagulant drugs or drugs known to increase the risk of hemorrhage, patients with a history of cerebrovascular disease (strokes) or brain tumors and patients who are not able to tolerate the prolonged stationary position during treatment also should not have the procedure.
ExAblate Neuro is manufactured by InSightec in Dallas, Texas.
For more information: www.insightec.com


 February 13, 2026
February 13, 2026 









