Image courtesy of Eizo Corp.
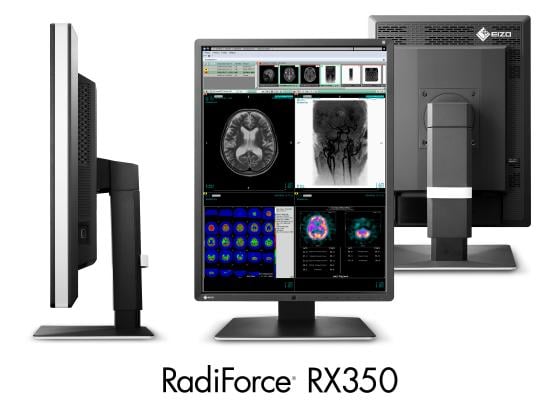
November 23, 2015 — Eizo Corp. released the RadiForce RX350, a 21.3-inch 3-megapixel monitor ideal for displaying chest X-ray, computed radiography (CR) and digital radiography (DR) grayscale images and color images such as 3-D rendering and image fusion.
The RadiForce RX350 is the successor model to the RadiForce RX340. The new monitor is equipped with Eizo’s Sharpness Recovery technology. All high-brightness LCD panels exhibit a decrease in sharpness of the original image due to aperture ratio of the pixels becoming larger. Sharpness Recovery restores lost information in contours, resulting in an image shown with maximum clarity.
A research study is being conducted on the RadiForce RX350 by Emory University to evaluate the user benefits of the Sharpness Recovery technology. Elizabeth A. Krupinski, Ph.D., who is spearheading the study commented, “Eizo is continuing to develop and introduce new and unique technology for the medical market. I am looking forward to evaluating Sharpness Recovery and seeing what it has to offer to radiologists.”
For keeping workspace efficient, the monitor’s width, height and depth were reduced by 22 mm, 39 mm and 45.5 mm respectively – a 30 percent difference compared to its predecessor. The thinner, black front bezels are ideal for viewing the screen in dark reading rooms, making it easier to focus on images, while the original white stripe design around the sides of the monitor presents a fresh, clean aesthetic to promote a comfortable, user-friendly environment.
Using the DisplayPort connection on the monitor, users can drive several monitors in a daisy-chain sequence. This allows users to configure a multi-monitor setup without the complicated hassle of excessive cabling.
RadiForce RX350 is currently pending U.S. Food and Drug Administration (FDA) 510(k) clearance and is not available for sale in the United States.
For more information: www.eizo.com


 January 22, 2026
January 22, 2026