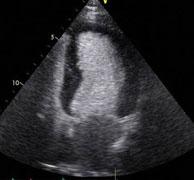
August 25, 2010 – Evidence suggests the use of contrast agents for echocardiography is safe and not associated with a greater incidence of myocardial infarction or mortality, according to a new study published in the American Journal of Cardiology (vol. 106, issue 5, pages 742-747, Sept. 1, 2010.)
In October 2007, the U.S. Food and Drug Administration (FDA) issued a black box warning for contrast agents used in patients undergoing echocardiography and restricted their use in patients with acute coronary syndrome, a decompensated heart and respiratory failure.
Owais A. Khawaja, M.D., department of internal medicine, Providence Hospital, Southfield, Mich., Kamran A. Shaikh, M.D., Henry Ford Health System, Detroit, and Mouaz H. Al-Mallah, M.D., Wayne State University, Detroit, performed a systemic review and meta-analysis to study the adverse effects of contrast agents used with respect to myocardial infarction and all-cause mortality. They used Medline, Embase, Biosis and Cochrane databases, searching for studies that reported myocardial infarction and all-cause mortality after the use of contrast agents for echocardiography.
Eight studies were included in the meta-analysis. A random-effect model was used and between-studies heterogeneity was estimated with I2. Eight studies reported death as an outcome and only four reported myocardial infarctions. The incidence of death in the contrast group was 0.34 percent (726 of 211,162 patients) compared to 0.9 percent (45,970 of 5.08 million patients) in the noncontrast group. The pooled odds ratio was 0.57 (95 percent confidence interval 0.32 to 1.01, p = 0.05). The reported incidence of myocardial infarction in the contrast group was 0.15 percent (86 of 57,264 patients) compared to 0.2 percent (92 of 44,503 patients) in the noncontrast group. The pooled odds ratio was 0.85 (95 percent confidence interval 0.35 to 2.05, p = 0.72). Significant heterogeneity was seen among the studies.
For more information: www.ajconline.org

 December 18, 2025
December 18, 2025 









