February 14, 2023 —DiA Imaging Analysis, a leading provider of advanced AI-based software solutions for ultrasound image analysis, announced today that the U.S. Food and Drug Administration (FDA) has cleared LVivo IQS, a new AI-based vendor-neutral software solution that enables ultrasound users to acquire high-quality interpretable cardiac ultrasound images that can be used to make better clinical assessments of the heart.
High-quality cardiac ultrasound image acquisition can be challenging due to the constant motion of the heart and its deep location in the chest, as well as the user expertise that varies between ultrasound users and settings.
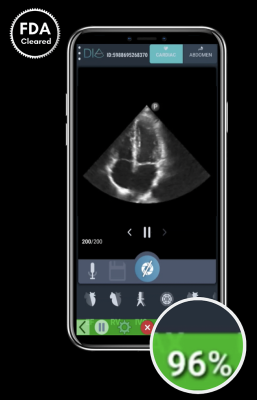
DiA’s new LVivo IQS - Image Quality Score provides real-time image quality feedback while scanning the left ventricle of the heart to assess the heart’s performance. The software uses colors and numerical scoring to indicate the quality level of the image as it is being scanned, helping users to produce optimal interpretable images for more accurate and reproducible analysis of the heart.
“LVivo IQS is the first of DiA’s 9 FDA-approved software solutions that empowers healthcare professionals with real-time feedback to enable the capture of high-quality ultrasound images,” said Hila Goldman-Aslan, CEO & Founder of DiA Imaging Analysis, “By harnessing the power of AI, our full suite of software solutions helps ultrasound users overcome two major challenges in the field – scanning high-quality images and accurately analyzing them. This holistic approach aligns with our mission of leveraging AI to make ultrasound image capture and analysis processes smarter and more accessible."
The FDA cleared LVivo IQS following a clinical study that demonstrated its safety and efficacy. The results demonstrated high agreement between the LVivo IQS AI’s quality score feedback and the ability to obtain clinically interpretable images as evaluated by Cardiologist specializing in echocardiography. 91% of images saved by point-of-care residents using LVivo IQS, were found to be clinically interpretable images by the Cardiologist.
“For precise heart diagnosis and treatment, high-quality cardiac ultrasound imaging is necessary in both point-of-care and other settings," said Dr. Lior Fuchs, Senior Intensive Care Physician at Soroka Medical Center in Israel and principal investigator of the LVivo IQS study.
"LVivo IQS AI software will assist us in obtaining faster and clearer images to aid our real-time clinical decision-making process.”
For more information: https://www.dia-analysis.com/


 February 13, 2026
February 13, 2026 









