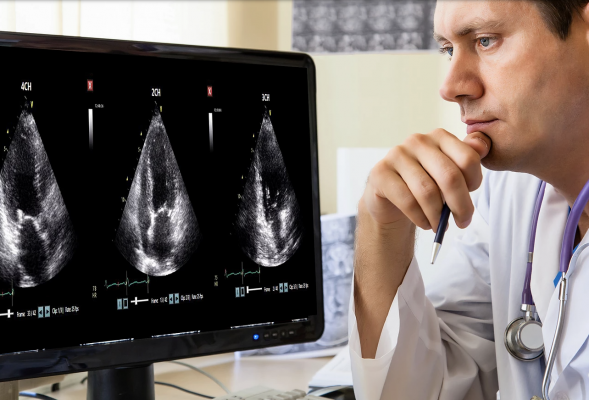
October 29. 2020 — DiA Imaging Analysis announced today that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the commercial use of LVivo Seamless for automatic view selection. LVivo Seamless algorithms automatically run "behind the scenes" to select the optimal cardiac ultrasound views out of 40-60 views in standard echocardiogram exams. Finding the right views in each echo exam is cumbersome and time-consuming. The ability to auto-select views is a breakthrough in AI implementation and the value that it brings to the echocardiography workflow.
"LVivo software operates without human touch, and adds great value," says Steve Feinstein, M.D., professor of cardiology at Rush University Medical Center. "These rapid, reliable and accurate serial views selections and measurements enable us to get second opinions and access anytime and anywhere."
The cleared LVivo Seamless solution integrates with DiA's other LVivo cardiac auto analysis toolbox products such as LVivo EF, an automated calculation of ejection fraction (EF) calculation, which is a main indicator of global heart function.
This enables clinicians to automatically obtain, for each echo-lab cardiac exam, optimal views and automated measurements in a fast, accurate and objective way.
All of DiA's ultrasound AI solutions are cross-platform and vendor-neutral; therefore, they can run on images acquired from any ultrasound device and can easily operate with any radiology image viewer.
"LVivo Seamless joins DiA's existing other solutions, to help reducing the variability associated with manually selecting views and visually analyzing cardiac ultrasound images," said Hila Goldman-Aslan, DiA's CEO and Co-Founder.
LVivo Toolbox provides both the clinical and financial value that stakeholders in the echo lab are looking for.
For more information: www.dia-analysis.com


 February 05, 2026
February 05, 2026 









