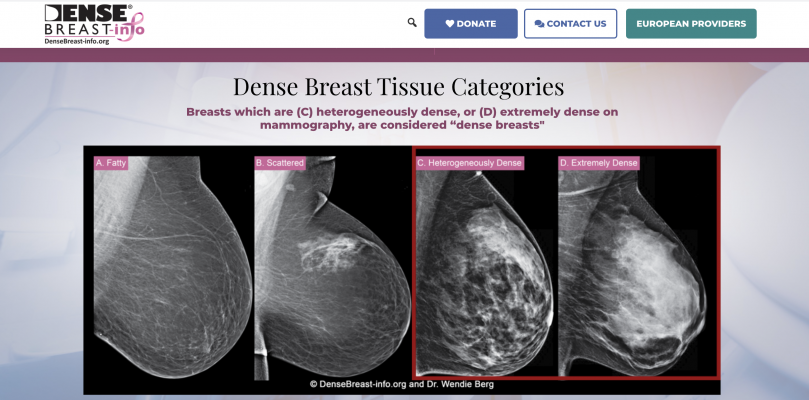
January 13, 2021 — DenseBreast-info.org (DB-I) invites health providers seeking the most up-to-date medically-sourced educational materials about the screening and risk implications of dense breast tissue and value of supplemental screening to visit the newly re-launched DB-I website at www.DenseBreast-info.org. Originally launched in 2015, the DB-I website has continued to evolve to meet the needs of providers and patients in the United States and around the world on the topic of breast density with expanded content and educational tools. In addition to featuring a new look, the DB-I website has been revamped to support a more user-friendly experience and address the increasing reach of the DB-I website, growing global interactions and to provide easier access to the substantial volume of information. New educational tools and resources include:
• Patient Resource Section – features downloadable education resources in 14 languages
• Expanded Spanish-Language Hub – updated screening guidance and FAQs
• State Density Inform Laws – new downloads and searchable Patient Inform and Insurance legislation
• DB-I published research/webinars
“As the conversation around breast density and personalized screening grows, the need for high quality medically sourced educational materials and tools has also increased. With more than 200,000 visits to the DB-I website in 2020 and nearly 300,000 projected for 2021, it is critical to continually add resources, as well as streamline the website to give providers quick and easy access to important content,” said JoAnn Pushkin, Executive Director, DenseBreast-info.
For more information: densebreast-info.org/


 February 18, 2026
February 18, 2026 









