
April 15, 2025 — CureMetrix, a provider of AI-driven medical imaging solutions, has announced that its AI-based cmAngio software received FDA clearance for use with GE Healthcare mammography imaging platforms. This builds on cmAngio’s initial FDA clearance for use with Hologic mammography imaging platforms.
FDA-cleared cmAngio is an AI-based software that reads mammograms and detects and localizes Breast Arterial Calcifications (BAC). cmAngio analyzes both full-field digital mammography (FFDM) and digital breast tomosynthesis (DBT) screening mammograms to accurately identify and mark these anomalies. It allows radiologists to use existing screening mammograms to identify women who have breast arterial calcifications and as appropriate refer them for additional evaluation.
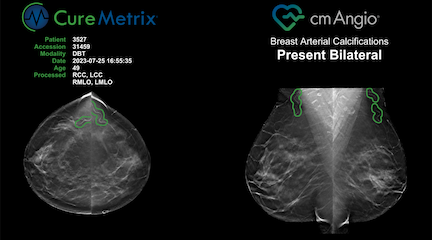 cmAngio enables a mammogram to serve as a two-for-one exam — screening for breast cancer while also detecting breast arterial calcifications. With this manufacturer expansion and FDA clearance, more radiologists can integrate cmAngio into their workflow, enhancing their ability to identify these incidental findings. This AI-powered advancement maximizes the clinical value of a single mammogram, providing women with important health insights without additional radiation or changes to standard radiology procedures.
cmAngio enables a mammogram to serve as a two-for-one exam — screening for breast cancer while also detecting breast arterial calcifications. With this manufacturer expansion and FDA clearance, more radiologists can integrate cmAngio into their workflow, enhancing their ability to identify these incidental findings. This AI-powered advancement maximizes the clinical value of a single mammogram, providing women with important health insights without additional radiation or changes to standard radiology procedures.
"This latest FDA clearance is a significant commercial milestone for CureMetrix," said Kevin Harris, President of CureMetrix. "By adding GE Healthcare to our list of validated imaging platforms, cmAngio expands its commercial footprint, reaching more radiologists in practices where GE is the primary mammography platform used. This broader compatibility supports our commitment to enhancing access for BAC detection across diverse clinical environments while increasing assurance that patients are receiving the highest standard of care available from screening mammography.”
CureMetrix customers are already preparing to implement this expanded capability across their networks. "We’re excited to launch cmAngio across SimonMed’s nationwide network of more than 150 imaging centers on May 5. Adding AI-enabled detection of breast arterial calcifications reinforces our commitment to seek out the most advanced and cutting-edge solutions for our patients. By equipping our radiologists with powerful AI, we’re advancing patient care and pioneering better clinical outcomes," said Dr. Sean Raj, Chief Medical Innovation Officer at SimonMed Imaging.
For more information about CureMetrix, cmAngio and its other women’s health solutions, please visit www.curemetrix.com.
For FDA clearance details, please visit: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K250754


 February 27, 2026
February 27, 2026 









