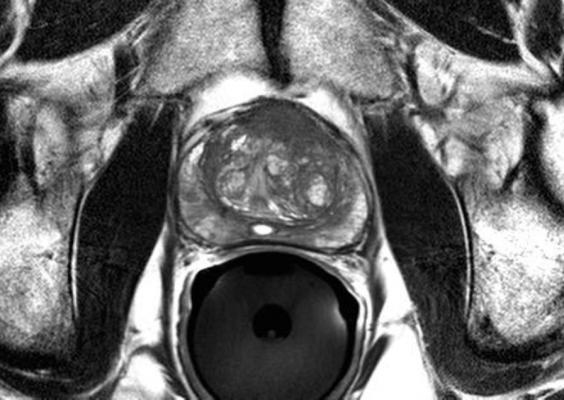
June 1, 2016 — A new Phase III clinical trial will look to evaluate if magnetic resonance imaging (MRI) can replace the current standard of care to diagnose prostate cancer. The primary objective of the multi-center PRECISE trial — sponsored by the Movember Foundation, the Ontario Institute for Cancer Research (OICR) and Prostate Cancer Canada — is to determine whether MRI can spare some men from undergoing a biopsy and avoid the possible associated side effects.
The trial will initially receive $3 million in funding. It will be led by Laurence Klotz, M.D., of the Sunnybrook Research Institute in Toronto.
MRI technology is a precise tool that could better identify which patients should undergo biopsy, and enable targeted biopsy of only areas suspected of malignancy. The PRECISE trial, which is estimated to be completed in three years, will investigate the ability of MRI to improve the diagnosis of clinically important disease and reduce the requirement for prostate biopsies.
Currently, prostate cancer is diagnosed by trans-rectal ultrasound (TRUS)-guided biopsy of the prostate, in most cases following a prostate specific antigen (PSA) test. TRUS-guided biopsy is associated with potential side effects such as infection and bleeding because it is not targeted, requiring numerous biopsy samples (between 10 and 12) to establish an accurate reading. In addition, this current standard of care is not sensitive enough to be able to discriminate between high-risk and very low-risk changes in prostate tissue, resulting in the over-diagnosis and over-treatment of many men, exacerbating the risk for side effects.
“If positive, this trial would support a change in practice from relying on biopsies for all men with suspected prostate cancer to providing MRI first with selective targeted biopsy,” explained Klotz. “This would allow 250,000 men per year in the U.S. and Canada to avoid unnecessary biopsies and the associated complications including hospitalization, without compromising our ability to identify clinically significant cancers.”
Data management and analysis for the trial will be conducted by the Ontario Clinical Oncology Group (OCOG) in the Escarpment Cancer Research Institute, a Hamilton Health Sciences and McMaster University institute.
For more information: www.prostatecancer.ca, www.oicr.on.ca


 February 13, 2026
February 13, 2026 









