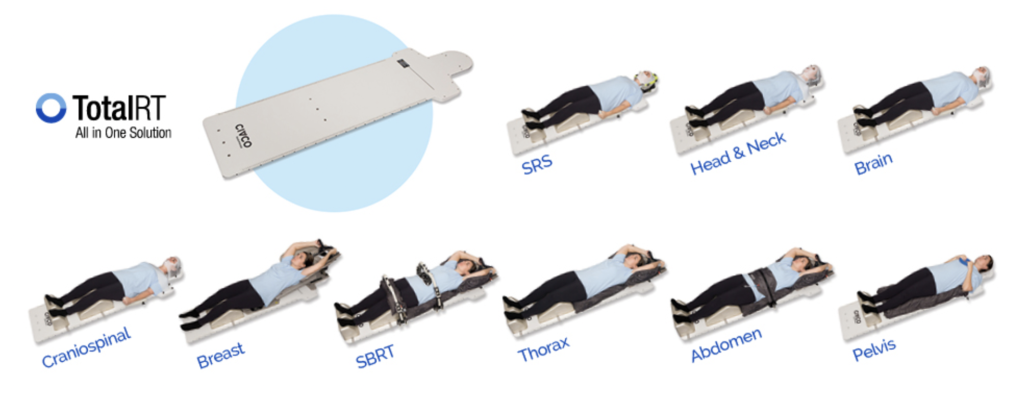
October 24, 2022 — CIVCO Radiotherapy, the leading global provider of high quality, innovative, patient-centric radiotherapy solutions, will feature the TotalRT All in One Solution along with several innovative solutions in booth #3766 at the ASTRO Annual Meeting in San Antonio, Texas, October 23-25, 2022.
The TotalRT Platform is designed to quickly adapt CIVCO RT's positioning and immobilization solutions to radiotherapy clinical setup and treatment needs, providing an all in one solution. The platform features a large, artifact-free and homogeneous treatment area for precise dose delivery and allows for repeatable patient positioning on CT, MR and photon treatment tables.
CIVCO RT will also display their recent innovations focusing on improving patient comfort and outcomes, including:
Evolve Breastboard
The innovative Evolve Breastboard is a modular and lightweight breast & thorax positioning solution that allows flexibility in patient setup without sacrificing comfort. The board's innovative GlideTrack technology allows quick and simple indexing of many Wing Boards, including the Pro Wing Board, to accommodate patients of varied torso lengths. Several hand grips, headrests and thermoplastic options easily integrate to maximize positioning options based on patient or clinical need.
Pediatric ProForm Head & Neck Solution
The Pediatric ProForm Universal Couchtop Extension, ProForm CT & MR Overlays, and thermoplastic options have been designed specifically for pediatric proton therapy. The extension design will allow attachment to current IBA, Hitachi and Mevion compatible pedestals. This pediatric solution compliments CIVCO RT's current adult ProForm series and is available for CT & MR imaging and proton & photon treatment.
Comfort Marker 2.0 Patient Marking System
The Comfort Marker 2.0 is the first and only commercially available marking device engineered to apply patient reference marks with gentle and controlled depth placement, resulting in less impact on the skin. The fast, sterile, and disposable needle module minimizes risk of needle stick incidents, providing patients and clinicians a safer environment.
Collaborative Partnerships
CIVCO RT encourages attendees to visit their booth to learn about many unique solutions offered by their collaborative partners, including Chabner XRT Radiation Bra and GrayDuck oral positioning stents.
For more information: www.civcort.com


 February 04, 2026
February 04, 2026 









