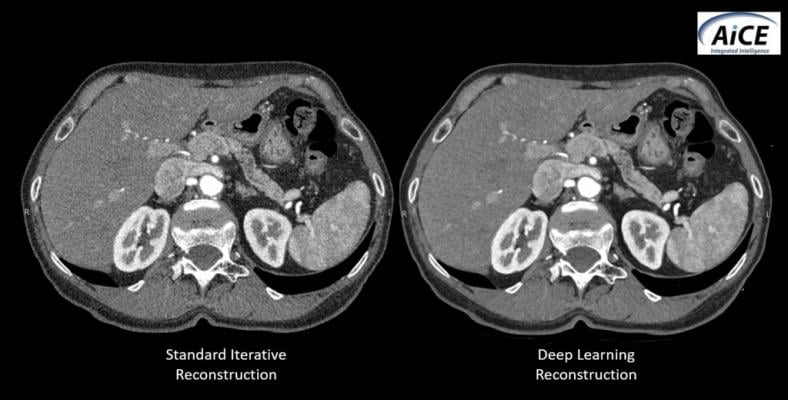
February 27, 2019 — Canon Medical Systems recently introduced AiCE (Advanced intelligent Clear IQ Engine), a deep convolutional neural network (DCNN) image reconstruction technology for computed tomography (CT). AiCE uses deep learning technology to differentiate signal from noise so that it removes noise while it preserves true signal.
With the AiCE deep learning approach, the DCNN is trained in the factory using perfect high-quality target data from real patient datasets. This patient data is extensively processed with advanced model based iterative reconstruction (MBIR), which provides optimal image quality and improved spatial resolution.
Following training and validation, the AiCE DCNN is then implemented into the CT scanner that allows for reconstruction speeds fast enough for busy clinical environments.
Canon Medical is showcasing its AiCE technology at this year’s European Congress of Radiology (ECR), Feb. 27-March 3 in Vienna, Austria.
For more information: www.us.medical.canon


 March 06, 2026
March 06, 2026 









