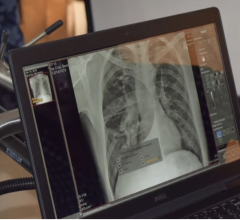
June 18, 2015 - Calgary Scientific Inc. announced the release of ResolutionMD 5.1. The latest software version enables physicians to make faster and more informed treatment decisions, by providing even greater access to medical images and information via Web and mobile devices.
Enhancements to the enterprise-image viewer include:
- A reengineered interface that enables quick and easy access to information regardless of browser in use;
- Client options which include HTML5 for Web and mobile, flex for the Web and native mobile applications;
- A discoverable tool set that ensures caregivers can gain quick access to the tools they need;
- Motion studies such as cardiology exams start automatically as the user loads them;
- Easier image comparison with multiple cine clips;
- Ability to instantly join collaboration sessions from any mobile device; and
- Seamless application launching from within Web-based or mobile applications.
The new functionality builds upon a recent release that included numerous new capabilities, including:
- Voice and video collaboration capability;
- Image sharing between facilities and providers;
- Integration capability to electronic medical record (EMR) systems;
- XDS integration;
- Precision measurements; and
- Extended non-DICOM support.
"We rely on ResolutionMD to get access to medical images safely and securely," said Kyle Hall, telehealth coordinator at Nebraska Medicine. "The combination of mobile image access along with ResolutionMD's new interface ensures our staff can look at data beyond the hospital walls so they can create quicker treatment plans and ultimately deliver better patient care."
ResolutionMD has U.S. Food and Drug Administration (FDA) Class II clearance for both the Web and mobile offering. With the recently announced FDA guidelines for mobile applications, FDA Class II clearance is now the required standard for any device being used for diagnosis or treatment decisions.
For more information: www.calgaryscientific.com


 February 10, 2026
February 10, 2026