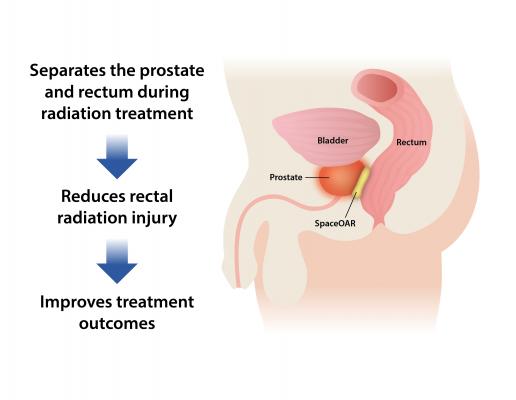
October 17, 2018 — Boston Scientific Corp. announced the close of its acquisition of Augmenix Inc., developer of the SpaceOAR Hydrogel System to help reduce common and debilitating side effects that men may experience after receiving radiotherapy to treat prostate cancer. The biodegradable SpaceOAR hydrogel is injected between the rectum and prostate to decrease a patient's exposure to rectal radiation and thereby reduce rectal radiation injury – one of the most common complications of prostate radiotherapy.
The close marks the third completed acquisition in 2018 for Boston Scientific’s Urology and Pelvic Health portfolio. The SpaceOAR Hydrogel System will expand the company’s prostate health portfolio, which includes therapies to treat benign prostatic hyperplasia, including the Rezūm System, the GreenLight XPS Laser Therapy System and holmium laser platforms.
Boston Scientific announced a definitive agreement to acquire Augmenix on Sept. 6, 2018 for $500 million in up-front cash and up to $100 million for reaching sales-based milestones.
For more information: www.bostonscientific.com


 February 04, 2026
February 04, 2026 









