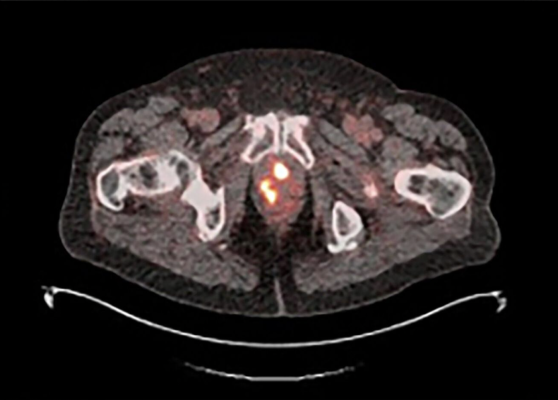
18F-rhPSMA-7.3 PET image showing multiple areas of uptake in the prostate gland in a man with newly diagnosed prostate cancer Photo courtesy of Blue Earth Diagnostics
December 2, 2022 — Blue Earth Diagnostics, a Bracco company and recognized leader in the development and commercialization of innovative PET radiopharmaceuticals, today announced topline results from its Phase 3 LIGHTHOUSE trial that evaluated the diagnostic performance and safety of 18F-rhPSMA-7.3 in newly diagnosed prostate cancer. 18F-rhPSMA-7.3 is an investigational high affinity radiohybrid (rh) Prostate-Specific Membrane Antigen-targeted PET imaging agent. The results were reported in a presentation at the 23rd Annual Scientific Meeting in Urologic Oncology (SUO), in San Diego, Calif.
“Effective staging of primary prostate cancer − determining its presence and whether it may have metastasized − is critical in assessing a patient’s prognosis and informing individual clinical management strategies,” said Brian F. Chapin, MD, Associate Professor, Department of Urology, Division of Surgery, The University of Texas MD Anderson Cancer Center, and Coordinating Investigator of the LIGHTHOUSE study. “Up to 25% of patients with primary prostate cancer may have detectable pelvic lymph node metastases, which are correlated with a risk for recurrence and associated overall survival. Conventional imaging techniques, such as MRI and CT, are limited in the information they may provide. Pelvic lymph node dissection (PLND), or pelvic lymphadenectomy, is considered the gold standard in assessing pelvic node lesions, but its use is limited to the planned surgical area. An ideal staging technique for detecting metastatic prostate cancer should include both pelvic nodes as well as more distant soft tissue and skeletal findings. The Phase 3 LIGHTHOUSE clinical study investigated the diagnostic performance of 18F-rhPSMA-7.3 PET imaging as a decision-making aid in assessing newly diagnosed prostate cancer in patients with unfavorable intermediate-, high- or very high-risk disease.”
“We are pleased to share these key Phase 3 LIGHTHOUSE study results with the urologic oncology community at SUO 2022, which are included in our New Drug Application for 18F-rhPSMA-7.3 PET imaging currently under review by the U.S. Food and Drug Administration,” said David E. Gauden, D.Phil., Chief Executive Officer of Blue Earth Diagnostics. “LIGHTHOUSE is the second of Blue Earth’s diagnostic imaging trials to report results based on novel radiohybrid technology PSMA technology, which offers potential theranostic utility in both diagnostic PET imaging and therapy. 18F-rhPSMA-7.3 represents a new class of PSMA-targeted PET radiopharmaceuticals, with early studies of 18F‐rhPSMA‐7.3 potentially showing a high binding affinity for PSMA, together with biodistribution data suggesting the potential for low bladder activity. Blue Earth Diagnostics is committed to helping men with prostate cancer across the care continuum, and we especially would like to thank the patients and clinical teams who participated in the LIGHTHOUSE study.”
The findings presented at SUO reported on the first results of the Phase 3 LIGHTHOUSE trial on the diagnostic performance and safety of 18F-rhPSMA-7.3 in men with newly diagnosed prostate cancer planned to undergo radical prostatectomy (RP). Co-primary endpoints were patient-level sensitivity and specificity of 18F-rhPSMA-7.3 PET for the detection of pelvic lymph node (PLN) metastases using histopathology as the standard of truth. The endpoints were evaluated for the Efficacy Analysis Population (EAP) of 296 patients who underwent 18F-rhPSMA-7.3 PET and had subsequent RP and PLN dissection. Based on the majority read from the three blinded, independent PET readers, the overall specificity of 18F-rhPSMA-7.3 PET/CT in the LIGHTHOUSE study was 96% (217/226). By majority read, the specificity was 95% (139/146) for high-risk or very high-risk, and 98% (78/80) for unfavorable intermediate-risk patients. The overall sensitivity of 18F-rhPSMA-7.3 PET/CT in the LIGHTHOUSE study was 24% (17/70) by majority read, which is consistent with reports to date of sensitivity within the class of PSMA-targeted diagnostic imaging radiopharmaceuticals. By majority read, the sensitivity was 27% (14/51) for high-risk or very high-risk, and 16% (3/19) for unfavorable intermediate-risk patients. No serious adverse events were observed in the LIGHTHOUSE study. Overall, 28 of the 356 (7.9%) patients in the Safety Population had at least one treatment-emergent adverse event that was considered possibly related to 18F-rhPSMA-7.3. The most frequently reported adverse event for patients in the Phase 3 LIGHTHOUSE study was injection site pain among 0.8% (3/356) of patients.
The LIGHTHOUSE Phase 3 clinical trial was a prospective, Phase 3, multi-center, single-arm, imaging study investigating the safety and diagnostic performance of 18F-rhPSMA-7.3 Positron Emission Tomography (PET) in men with newly diagnosed prostate cancer. The study enrolled 356 patients at clinical sites in the United States and Europe. Additional information about the Phase 3 LIGHTHOUSE trial is available at www.clinicaltrials.gov (NC04186819).
The findings, “Diagnostic Performance and Safety of 18F-rhPSMA-7.3 PET in Patients with Newly Diagnosed Prostate Cancer: Results from a Phase 3, Prospective, Multicenter Study (LIGHTHOUSE),” were presented at SUO 2022 on December 1, 2022, by Brian F. Chapin, MD, Associate Professor, Department of Urology, Division of Surgery, The University of Texas MD Anderson Cancer Center, on behalf of the LIGHTHOUSE Study Group. Full session details and the abstract are available in the SUO online program here.
About Radiohybrid Prostate-Specific Membrane Antigen (rhPSMA)
rhPSMA compounds consist of a radiohybrid (“rh”) Prostate-Specific Membrane Antigen-targeted receptor ligand which attaches to and is internalized by prostate cancer cells and they may be radiolabeled with 18F for PET imaging, or with isotopes such as 177Lu or 225Ac for therapeutic use – creating a true theranostic technology. They may play an important role in patient management in the future, and offer the potential for precision medicine for men with prostate cancer. Radiohybrid technology and rhPSMA originated from the Technical University of Munich, Germany. Blue Earth Diagnostics acquired exclusive, worldwide rights to rhPSMA diagnostic imaging technology from Scintomics GmbH in 2018, and therapeutic rights in 2020, and has sublicensed the therapeutic application to its sister company Blue Earth Therapeutics. Blue Earth Diagnostics has completed two Phase 3 clinical studies evaluating the safety and diagnostic performance of 18F-rhPSMA-7.3 PET imaging in prostate cancer: (“SPOTLIGHT,” NCT04186845), in men with recurrent disease and (“LIGHTHOUSE,” NCT04186819), in men with newly diagnosed prostate cancer. Currently, rhPSMA compounds are investigational and have not received regulatory approval.
For more information: www.blueearthdiagnostics.com


 February 13, 2026
February 13, 2026 









