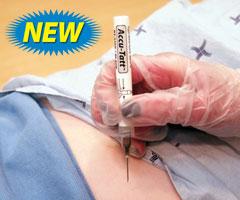
January 31, 2013 — Accu-Tatt is an easy-to-use, sterile, all inclusive tattooing device designed to permanently mark radiation therapy patients. Every Accu-Tatt pouch conveniently contains an ink ampoule and a ¾-inch, 22-gauge needle. Packaging the needle and ampoule in a sterilized pouch reduces the concern for contamination and cross-contamination within radiation therapy facilities. In addition, Accu-Tatt’s short needle length allows for increased control while tattooing the patient. The Accu-Tatt shell contains an ampoule filled with sterile black ink. To start the flow of ink, hold the ampoule upright in one hand and use the thumb to bend the shell, breaking the ampoule. The end of the ampoule houses a luer lock fitting to accommodate the prepackaged, sterilized hypodermic needle.
Twenty-five individually wrapped Accu-Tatt systems will be packaged together in a dispensing box. Each pouch, containing the needle and ampoule, is sterilized.
Permanent tattoos are used during radiation therapy to mark the edge of the treatment field. These permanent markings help ensure the reproducibility of the patient throughout the treatment cycle.
The product is available now for immediate purchase.
For more information: www.bionixrt.com


 January 30, 2026
January 30, 2026 









