December 22, 2009 – The Baylor University Medical Center in Dallas has taken delivery of the Naviscan PEM (positron emission mammography) scanner at its Darlene G. Cass Women’s Imaging Center.
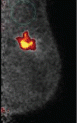
Baylor will utilize PEM scanner to complement its existing anatomical tools by providing a critical 3-D metabolic perspective of breast cancer. The metabolic view allows physicians to distinguish between benign and malignant lesions. A recent multicenter NIH-sponsored study comparing PEM and MRI highlighted that PEM had improved specificity relative to MRI at comparable sensitivity.
The Darlene G. Cass Women’s Imaging Center has been a leading breast imaging service in the Dallas area for more than 20 years, performing more than 50,000 breast imaging procedures annually.
“We recently received prestigious designation as one of five ‘Top Women’s Imaging Centers to Watch in 2009’ from Imaging Technology News acknowledging our commitment to providing the highest level of breast care to our patients,” said Zeeshan Shah, M.D., at Baylor University. “Our leading technology, radiology expertise and our dedication to the detection of breast cancer has allowed us to provide exquisite care to thousands of women. Our expectation is that PEM will prove indispensable to effective breast cancer management for both our referring physicians and our patient population.”
The Naviscan PEM scanner produces high-resolution tomographic images at 2 mm resolution, allowing physicians to visualize breast tumors about the size of a grain of rice. The scanner is the size of a mammography unit and consists of two high-resolution detector heads which are placed in close proximity to the breast. Compared to the higher-force compression necessary for mammography, the Naviscan PEM scanner uses gentle breast immobilization.
For more information: www.naviscan.com


 February 17, 2026
February 17, 2026 









