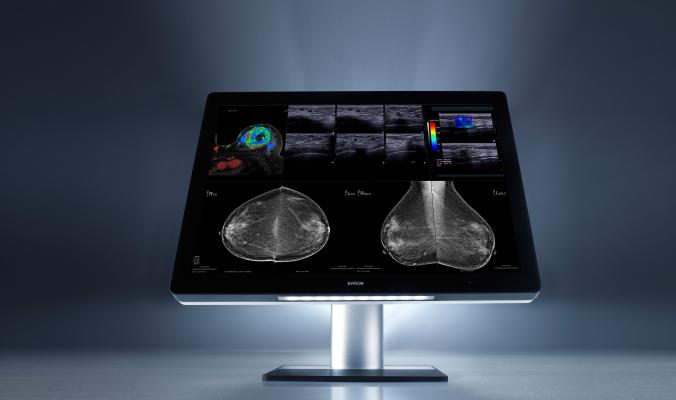
Coronis Uniti image courtesy of Barco
September 17, 2015 — Healthcare imaging specialist Barco announced it has received the 2015 Frost & Sullivan Award for best practices in Visionary Innovation Leadership in the medical imaging displays market. The award comes on the heels of the release of the Coronis Uniti display system in 2014.
“Coronis Uniti is an evolutionary platform, a universal imaging display system that can support every type of procedure across imaging modalities,” commented David Frigstad, Frost & Sullivan’s chairman. “The product reflects a visionary understanding of the future which is why Frost & Sullivan decided to recognize Barco as the Visionary Innovation Leader in the global medical imaging displays market.”
Coronis Uniti is the only display that can support picture archiving and communication systems (PACS) as well as multimodality breast imaging — i.e. 3-D mammography, digital mammography, breast magnetic resonance imaging (MRI) and ultrasound — on a single screen. This provides an alternative to multi-monitor workstation configurations and eliminates the need to change workstations to complete patient studies. Its 33-inch screen with 12-megapixel resolution renders high-quality grayscales as well as color and fused modalities, in 2-D and 3-D, static and dynamic.
“Coronis Uniti has the potential to elevate a healthcare organization’s diagnostic viewing environment and contribute actively to meeting many of the high-level objectives sought by these organizations, such as enabling earlier detection of pathologies, gaining more confidence in final diagnoses and reducing the rate of patient recalls,” Frigstad concluded.
Frost & Sullivan also applauded Barco for its significant contributions to the educational and lobbying efforts necessary to advance the regulatory environment and the clinical adoption curve. In the case of Coronis Uniti, Barco has been actively participating in industry efforts to establish standards for the use of color in medical imaging and has been taking a leadership role in defining the color standard display function (CDSF).
For more information: www.barco.com


 January 22, 2026
January 22, 2026