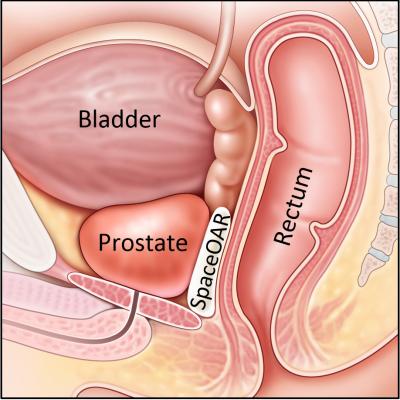
June 28, 2017 — Augmenix Inc. announced that the first patient in New Zealand has been treated with SpaceOAR hydrogel at the Kathleen Kilgour Centre (KKC) in Tauranga. SpaceOAR hydrogel is the first absorbable spacer designed to separate the rectum and prostate to reduce the risk of long-term side effects after radiation treatment.
Unintended injury to surrounding tissue during radiation therapy can lead to bowel, urinary and sexual symptoms that can affect patient health and quality of life (QOL). With SpaceOAR hydrogel, physicians can help reduce this risk by placing a hydrogel barrier to separate the prostate from adjacent healthy tissue. SpaceOAR hydrogel is initially implanted as a liquid that solidifies into a soft hydrogel that pushes the prostate and rectum apart. It remains stable for three months during radiation therapy and then is gradually absorbed and eliminated by the body.
In January 2017, Augmenix announced three-year post-treatment data from a prospective, randomized, multi-center, patient-blinded clinical trial showing that patients treated with SpaceOAR hydrogel prior to prostate cancer radiotherapy demonstrated significant rectal (bowel), urinary and sexual benefit through three years of follow up. During radiotherapy, the spacer resulted in a 73.5 percent reduction in rectal V70 radiation dose and a 49 percent reduction in median penile bulb radiation dose in patients treated with SpaceOAR hydrogel compared to men who did not receive the hydrogel (control). Overall patient wellness at three years was assessed by looking at the percent of patients with clinically significant declines in bowel, urinary and sexual QOL domains combined. Three years after radiotherapy, patients that were not treated with SpaceOAR hydrogel were 8 times more likely to experience significant declines in all three QOL areas (p=0.002). Among men who were sexually potent at baseline, the analysis showed that men in the SpaceOAR hydrogel arm were better able to maintain erections sufficient for intercourse through 3 years of follow-up (p=0.03). Of these men, 66.7 percent were able to achieve erections sufficient for intercourse at three years compared to 37.5 percent in the control arm, a 77.8 percent relative improvement.
“KKC is dedicated to providing the best care for our patients and the introduction of SpaceOAR hydrogel for men with prostate cancer is yet another step in achieving this,” said Leanne Tyrie, MBChB, genitourinary radiation oncologist and clinical director at the Kathleen Kilgour Centre. “The significant decrease in bowel, urinary and sexual side effects following radiotherapy when SpaceOAR hydrogel is utilised, made our decision to incorporate it as part of standard of care for prostate cancer patients very easy.”
SpaceOAR hydrogel is U.S. Food and Drug Administration (FDA)-cleared and CE marked.
For more information: www.augmenix.com


 February 04, 2026
February 04, 2026 









