Aug. 10, 2017 — The American College of Radiology (ACR) developed three new guidance documents and revised 32 others to advance the science of radiology and improve the quality of service to patients.
“Each practice parameter and technical standard represents a policy statement by the ACR. Each document is an educational tool that addresses the indications, technique, personnel qualifications, and recommended methods of interpretation and documentation specific to its topic,” said Matthew Pollack, M.D., FACR, chair of the ACR Committee on Practice Parameters and Technical Standards. “Every practice parameter and technical standard undergoes an extensive review process prior to final approval for publication and dissemination,” he noted.
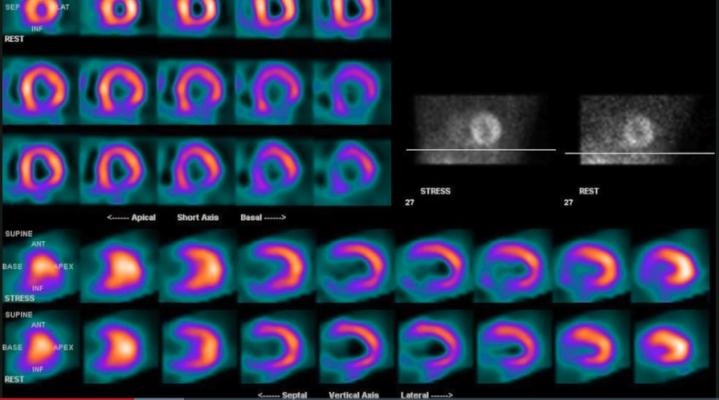
The two new multi-society practice parameters address the performance of dopamine transporter SPECT imaging for movement disorders and cardiac PET/CT imaging. The new multi-society Technical Standard focuses on diagnostic procedures using radiopharmaceuticals.
The revised documents cover imaging modality, organ or body system and radiology subspecialties. The guidance documents are posted on the ACR website and take effect Oct. 1. All ACR Practice Parameters and Technical Standards are available at no charge.
For more information: www.acr.org


 February 04, 2026
February 04, 2026 









