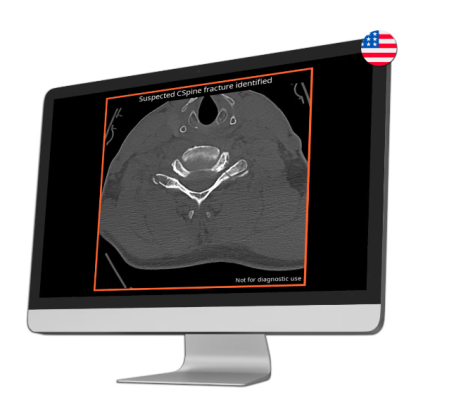
Sept. 19, 2024 – Medical imaging AI company Avicenna.AI recently announced it had received 510(k) clearance from the U.S. Food and Drug Administration for its CINA-CSpine tool.
CINA-CSpine is an AI tool designed to support the detection and triage of cervical spine fractures from CT images.
While the prognosis for cervical spine fractures depends on the severity of the injury, the timeliness of treatment is key, so prompt detection and appropriate treatment are crucial for optimizing outcomes and minimizing long-term complications. CINA-CSpine automatically flags suspected positive findings compatible with acute cervical spine fractures, seamlessly alerting radiologists through their existing systems.
“Cervical spine fractures are serious injuries that require prompt and appropriate medical attention, especially if the spinal cord is involved, so accurate diagnosis is essential,” said Cyril Di Grandi, co-founder and CEO of Avicenna.AI. “With CINA-CSpine, we aim to help reduce the delay between scan and interpretation, which is critical in the treatment of this condition.”
CINA-CSpine was validated on more than 300 non-contrast CT scans provided by multiple sources in the U.S. and Europe, and acquired from 36 different scanner models across five vendors. The device achieved an overall sensitivity and specificity of 90.3 percent and 91.9 percent, respectively, when compared to the ground truth established by the consensus of three US-board certified senior radiologists.
“These results demonstrate that our CINA-CSpine algorithm is capable of providing prompt and accurate findings that could positively guide physicians towards better assessments and improved patient outcomes,” added Di Grandi.
Additional information is available at www.avicenna.ai.


 February 20, 2026
February 20, 2026 









