May 11, 2022 — Adaptiiv Medical Technologies is collaborating with HP Inc. and Varian, a Siemens Healthineers company (Varian), to advance the quality of and access to personalized cancer care for U.S. patients with 3D printed medical devices.
HP's Multi Jet Fusion platform provides access to leading 3D printing technology that is used to produce high-quality, flexible, patient-specific parts at high throughput. HP 3D printing is suitable for use in healthcare because of the speed, quality, and economic advantages gained through HP's scalable manufacturing processes.
"The combined technology scale of HP and Varian, along with the leading-edge personalization workflow of Adaptiiv, provides the improved solution that clinicians and patients deserve," said Louis Kim, Vice President, 3D Printing at HP. HP is proud to be a part of this collaboration to help advance the treatment of cancers worldwide."
Adaptiiv will also work with Varian to expand access to personalized, patient-specific 3D printed medical devices. For more than 70 years, Varian has developed, built, and delivered innovative cancer care technologies and solutions for clinical partners around the globe to help them treat millions of patients each year. This agreement advances Varian's commitment to meeting evolving customer needs and improving the patient experience.
"We are energized by Adaptiiv's innovation and this opportunity with HP to further expand our cancer care ecosystem. Addressing our customers' needs is our top priority, and we are confident that this commercial commitment will help us do just that by advancing interoperability with Adaptiiv's 3D printed medical devices for external beam radiation therapy," said Ben Moga, Director, Strategic Alliances and Synergy Investments at Varian.
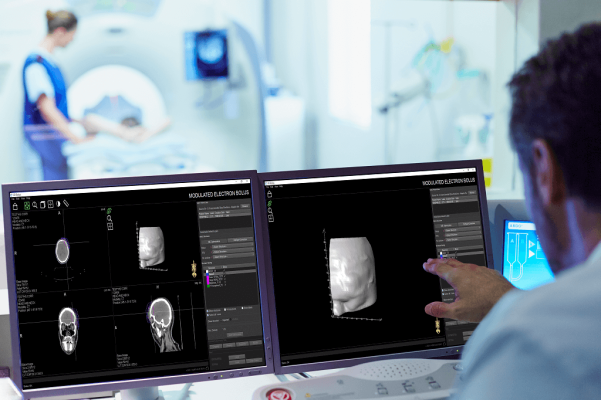
Earlier this year, Adaptiiv received U.S. FDA 510(k) clearance for the Adaptiiv On Demand service to manufacture and deliver 3D printed patient-specific medical devices. 3D printed medical devices conform to patient anatomy thereby improving the accuracy of dose delivery while the integration of Adaptiiv software into clinical workflows provides improved efficiencies, such as reducing patient set up time both in CT simulation and on the treatment unit.
"This is a tremendous milestone for Adaptiiv's vision to democratize personalization in radiation treatment," said Adaptiiv CEO, Alex Dunphy. "Collaborating with brands like HP and Varian who stand for quality and innovation will ensure our solutions reach patients around the world. The last mile of radiation therapy needs to evolve and our solutions provide greater access to personalized care, while improving treatment and creating workflow efficiencies for cancer centres around the world."
For more information: www.adaptiiv.com


 February 04, 2026
February 04, 2026