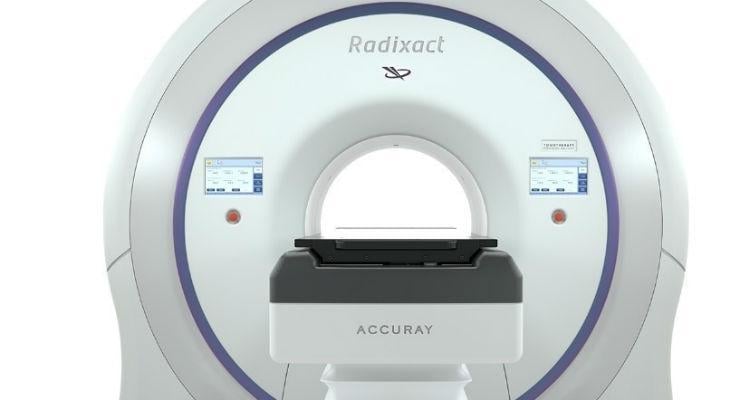
February 13, 2017 — Accuray Inc. announced that it has received Shonin approval from the Japanese Ministry of Health, Labor and Welfare (MHLW) to market its Radixact Treatment Delivery System, Accuray Precision Treatment Planning System and iDMS Data Management System. Together, the treatment delivery system and software solutions enable clinicians to provide efficient, highly precise treatment for a wider range of cancer patients, including those undergoing retreatment. This new Radixact platform is now available in Japan, in certain markets in the European Union, and in the U.S. market.
The Radixact System was designed to be an easy-to-operate, highly reliable, advanced radiation therapy device. The system features a more powerful linear accelerator, low-dose fan beam megavoltage computed tomography (MVCT) imaging and unique helical treatment mode, so that clinicians can deliver highly accurate, individualized dose distributions to any tumor. The fully-integrated, simplified adaptive treatment planning solution enables clinicians to routinely adjust treatment plans during the course of treatment to account for changes in tumor size, shape and location – thereby increasing treatment precision.
For more information: www.accuray.com/radixact


 January 30, 2026
January 30, 2026 









