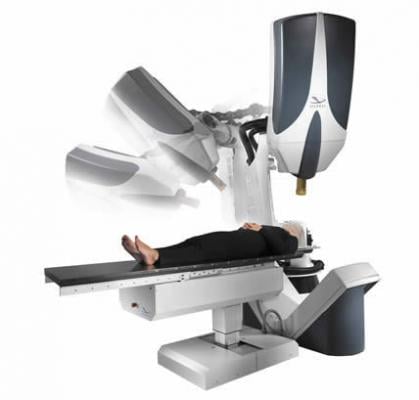
March 6, 2012 – Accelitech and partners have just completed a transaction to bring the first and only
CyberKnife stereotactic radiosurgery system to the Republic of Ireland. Scheduled to begin treating
patients in fall of 2012, the center will open on the campus of the Hermitage Medical Clinic in Dublin.
The partnership includes the following parties: Accelitech (lead developer), Neurosurgery Ireland (a
prominent Irish neurosurgical group), Regent Surgical Health (a developer of high-end specialized
surgical programs), the Hermitage Medical Clinic and several other local providers, all of whom share
the vision and interest in bringing this state-of-the-art cancer treatment technology to the people of
Ireland.
Historically, Irish patients requiring CyberKnife radiosurgery treatments—and in many cases their
treating neurosurgeons — have been required to travel to other countries to complete treatments.
"This partnership is a big win for the people of Ireland," says Scott Milligan, chief development officer
for Accelitech. "The new CyberKnife program will combine the very best clinical teams in the country
with the latest advanced technology in a conveniently located, patient-focused facility."
For more information: www.accelitech.biz


 January 30, 2026
January 30, 2026 









