August 2, 2013 — A “test run” of radiation therapy in patients with non-Hodgkin lymphoma can show how much radiation is likely to be absorbed by a tumor during actual treatment. This information may help doctors to estimate the dose needed for effective treatment more precisely than currently used measures, such as a person’s height and weight. Ensuring proper radiation dosages reduces damage to healthy tissues and may also prevent tumor growth for a longer period of time.
Before beginning radioimmunotherapy, patients are injected with a very small amount of the radiolabeled antibody for an imaging scan. Because the antibodies go directly to cancer cells, imaging technologies that pick up radiation, like single-photon emission computed tomography (SPECT), can show where tumors are located. Doctors then consider the patient’s height and weight, how quickly a particular radioactive marker is cleared from the body or other factors to calculate the radiation dose that will be used during treatment.
Even with careful planning, however, doctors and patients cannot be certain how a particular tumor will respond to radiation until treatment begins.
National Institute of Biomedical Imaging and Bioengineering (NIBIB) grantee Yuni Dewaraja, Ph.D., and her research team at the University of Michigan sought to find out if the pre-treatment scan could predict tumor shrinkage and progression-free survival — how long a patient lived without further tumor growth. If so, radiation treatment plans could be based on personalized patient tumor data rather than more general guidelines.
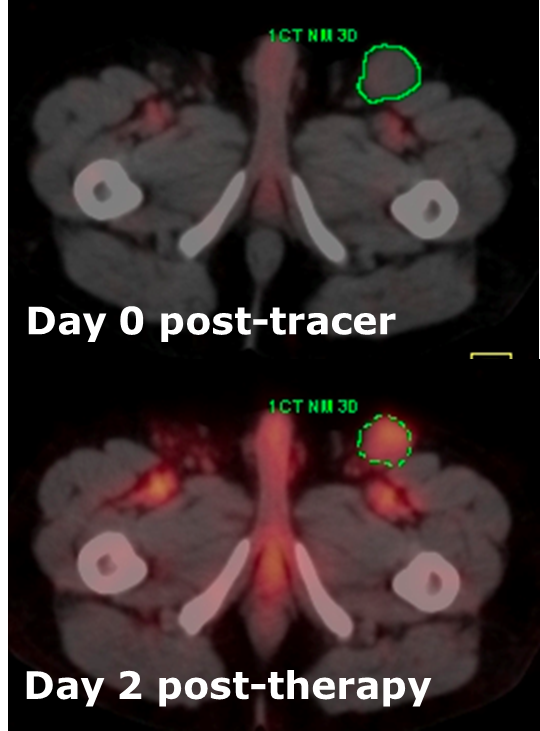
To explore these questions, the researchers assessed 130 B-cell non-Hodgkin tumors in 39 study participants. The participants received a pre-treatment dose of a radio-labeled antibody — specifically, 131I-tositumomab — and then underwent anatomic computed tomography (CT) and SPECT on a combined SPECT/CT system.
From these scans, an advanced computer program created detailed 3-D pictures showing the pattern of radiation absorption throughout a particular tumor. The programs for SPECT image reconstruction and absorbed dose calculation were also developed by this group with past funding from NIBIB.
Eight days after pre-treatment, the participants received their full treatment doses. To evaluate outcomes, they were re-scanned two and six months after treatment, and then every six months afterward for up to four years. For the follow-up scans, the researchers used a different imaging system combining CT with positron emission tomography (PET), which produces higher resolution images than SPECT/CT but can’t be used in radioimmunotherapy for this type of cancer because it does not detect the radiation emitted by 131I-tositumomab.
The researchers considered a range of factors likely to affect treatment outcomes, including size of the tumor before treatment, number of past treatments, and the presence of genes that may make the tumor cells more resistant to radiation. Of all the factors studied, the one most closely associated with progression-free survival was the amount of radiation absorbed by a tumor, which was predicted by the pre-treatment scan.
Further, the researchers found that a dose of at least 200 centigray (cGy, a unit of radiation absorption) to the tumor provided longer periods of progression-free survival. On average, participants whose tumor absorbed 200 cGy or more survived nearly 14 months without additional tumor growth, while those whose tumor absorbed less than 200 cGy experienced only about 2 months without tumor growth.
For more information: www.nibib.nih.gov


 January 30, 2026
January 30, 2026 









