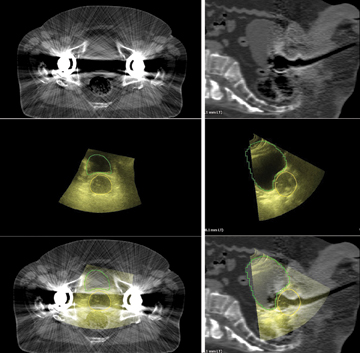
Axial (left column) and sagittal (right column) images of a patient with bilateral hip prostheses. Top images (Figure 1) are 3-D CT, middle (Figure 2) are Clarity 3-D ultrasound images with the prostate and bladder contoured, and bottom images are the fused 3-D datasets.
Soft tissue imaging in radiation therapy planning and delivery is relatively new and has been spawned by the development of linear accelerator-mounted kV imaging devices, as well as improvements in ultrasound technology. We have used Clarity 3-D ultrasound for target definition and daily target localization for breast and prostate cancers for the last four years and are rapidly moving toward daily, continuous monitoring of prostate gland position using transperineal ultrasound (TPUS), a technique we helped develop. We are continuing a series of clinical trials to evaluate TPUS in the treatment of prostate cancer.
We first confirmed the feasibility of this technique and are currently evaluating its use in target imaging, specifically in comparison to magnetic resonance imaging (MRI). Our ongoing work evaluates TPUS with respect to image reproducibility through the course of therapy, and ultimately we plan to assess its use on a daily basis to track the prostate gland during treatment.
Target Imaging Beyond CT
Computed tomography (CT)-based treatment planning is standard for the treatment of radiation targets adjacent to critical structures, particularly when high radiation doses are required to maximize the potential for local control. A radiation dose-response relationship clearly exists for prostate cancer[1], and advances in radiation technique are often adapted rapidly in an effort to safely delivery higher doses of radiation treatment without exceeding normal radiation tolerances of the adjacent bladder and rectum.
From the earliest days of CT-based treatment planning for prostate cancer, it has been understood that the CT-derived treatment volumes overestimate the actual volume of the prostate by up to 35 percent.[2] It is argued that our historical favorable local control data are based on treating these artificially expanded volumes. However, highly conformal techniques — such as prostate seed brachytherapy — use more accurate ultrasound images of the gland, and our long-term results treating the smaller (and true) treatment volumes are excellent.[3]
It is logical to assume that external beam approaches can safely use the most accurate (and smaller) target volumes, provided they are accounted for in the radiation treatment planning process. MRI provides a more accurate definition of the prostate contour than CT[4], however its routine use is costly and it has not been shown to improve on our current, CT-based treatment planning systems.
At the University of Vermont/Fletcher Allen Health Care, we have adopted the routine use of transabdominal ultrasound/CT image fusion in the treatment planning process. This approach has been particularly helpful in patients who present challenges to standard imaging, including those with metal prosthetic hip arthroplasties (Figure 1). In our ongoing comparison trial of Clarity Autoscan with TPUS compared to MRI for image fusion, early experience suggests that a TPUS obtained in the simulation position and acquired simultaneously with the planning CT is superior to an MRI obtained on a different machine, at a different time with different amounts of bladder and rectal filling.
Daily Targeting Possibilities
Image-guidance for the treatment of prostate cancer has been adopted as the standard of care with good reason. Careful localization of the prostate gland prior to daily treatment leads to better control with less toxicity.[5] Initial experience with 2-D B-mode acquisition ultrasound suggested that transabdominal imaging of the prostate gland on a daily basis was possible, but the reproducibility of that technique was limited.
This initial attempt at ultrasound-based image-guided radiotherapy (IGRT) used CT, rather than ultrasound to determine the initial target reference volume. The 2-D approach also limited the imaging capabilities of this approach.
The Clarity ultrasound system uses ultrasound at the time of simulation to identify the ultrasound-visible reference volume. The 3-D image acquisition permits simultaneous image reconstruction in three planes and offers good reproducibility when compared to other IGRT modalities.
Our current practice is to use both fiducial-based and 3-D ultrasound IGRT techniques on most men (typically one modality daily and both on the same day once a week). Our experience suggests that the small differences we typically see when scans using both modalities are performed on the same day are more likely related to target motion between image acquisition rather than significant differences in modality accuracy.
We do observe limitations in ultrasound imaging in some patients (poor bladder filling, large body habitus). But our experience, based on thousands of ultrasound IGRT sessions, suggests that most of these limitations are overcome with consistent techniques and excellent training of the therapy staff.
We have been involved in the development of a new TPUS 3-D ultrasound approach to prostate image guidance. We completed an initial trial of 15 patients using transperineal probe placement, and over the course of that experience, helped develop a robust applicator, probe and software techniques.
The transperineal approach puts the probe closer to the gland than other ultrasound techniques, does not depend on bladder filling and enables its use during standard intensity-modulated radiotherapy (IMRT)/volumetric modulated arc therapy (VMAT) approaches. The new TPUS images are typically clear, the external contours easily visible and continuous scanning allows for automated segmentation techniques that will facilitate concurrent treatment and imaging (Figure 2).
Real-Time Tracking
Standard radiation treatment delivery provides reasonable chances of local control with tolerable risks of side effects. With advances in imaging and treatment delivery, there is emerging interest in hypofractionated radiation approaches.
The theoretical advantages of this approach are intriguing, and the radiobiology may ultimately be proven to be favorable. But many institutions as well as cooperative groups require or recommend some attempt to track target motion during hypofractionated treatment delivery, particularly if treatment times are expected to exceed five minutes.
As prolonged treatment times are associated with more potential for prostate motion, the ideal tracking solution would allow simultaneous imaging and treatment. Most fiducial-based IGRT systems require treatment interruption to acquire images, as well as a subsequent process (automated or manual) to compare the images obtained to a previously obtained reference image. Providing the time necessary for these tasks prolongs treatment time and increases the potential for prostate gland movement.
Implanted electromagnetic transponders inserted into the prostate gland can be tracked within an electromagnetic array. However, these serve only as surrogates of the true prostate volume and are subject to significant and variable changes in position as the prostate volume changes during the course of radiation treatment.[6]
Ultrasound imaging of the entire prostate on a daily basis enables evaluation of the gland and its position relative to the surrounding structures. In our use of transabdominal ultrasound, we do encounter changes in prostate shape and size during the course of therapy (most notably in those men on androgen deprivation), as well as rotations about the axis due to variable and asymmetric filling of the rectum. We can and do accommodate for these changes daily during a course of therapy and will continue to do so as we increase our utilization of transperineal ultrasound.
Conclusion
TPUS imaging techniques provide excellent prostate gland imaging and permits visualization of the gland during radiation treatment. We continue our evaluation of TPUS as a tool to assist in prostate volume definition at the time of simulation. We are rapidly moving toward a formal evaluation of TPUS during treatment in an effort to control for intrafraction motion of the gland and anticipate its routine use, particularly with hypofractionated treatment regimens.
H. James Wallace, M.D., is the medical director of radiation oncology at Fletcher Allen Health Care (FAHC). His areas of expertise include genitourinary cancer, lymphoma, pediatric cancer and radiosurgery. He also is an associate professor at the University of Vermont College of Medicine.
References:
1. Eade, T.N. et al, “What dose of external-beam radiation is high enough for prostate cancer?” Int J Rad Onc Biol Phys, 2007. 68(3): p. 682-9.
2. Roach, M., 3rd et al, “Prostate volumes defined by magnetic resonance imaging and computerized tomographic scans for three-dimensional conformal radiotherapy.” Int J Rad Onc Biol Phys, 1996. 35(5): p. 1011-8.
3. Sylvester, J.E. et al, “Fifteen-year biochemical relapse-free survival, cause-specific survival, and overall survival following I(125) prostate brachytherapy in clinically localized prostate cancer: Seattle experience.” Int J Rad Onc Biol Phys, 2011. 81(2): p. 376-81.
4. Hentschel, B. et al, “Definition of the CTV prostate in CT and MRI by using CT-MRI image fusion in IMRT planning for prostate cancer.” Strahlenther Onkol, 2011. 187(3): p. 183-90.
5. Zelefsky, M.J. et al, “Improved Clinical Outcomes with High-Dose Image Guided Radiotherapy Compared with Non-IGRT for the Treatment of Clinically Localized Prostate Cancer.” Int J Rad Onc Biol Phys, 2012.
6. King, B.L. et al, “Electromagnetic transponders indicate prostate size increase followed by decrease during the course of external beam radiation therapy.” Int J Rad Onc Biol Phys, 2011. 79(5): p. 1350-7.


 February 05, 2026
February 05, 2026 









