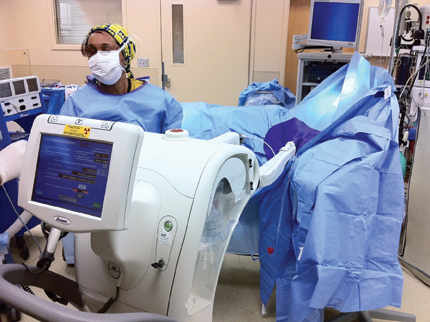
An actual intraoperative delivery a Xoft unit is attached to a balloon ready for treatment.
Intraoperative radiation therapy (IORT) for breast cancer is emerging as a newer alternative to whole-breast radiation therapy and accelerated partial breast irradiation (APBI). The Axxent eBx system from Xoft is a proprietary electronic brachytherapy platform designed to deliver 50 kV radiation, with minimal intraoperative shielding requirements. It becomes a functional and convenient modality for the delivery of intraoperative radiation therapy (IORT).
IORT is one method that might offer advantages over conventional APBI techniques. There is the advantage of excellent delineation of the tumor bed under visual control, very good dose distribution and high sparing of the normal tissue. Recent randomized trials showing excellent tumor control have generated new interest in IORT. These advantages were one of the goals of the TARGIT-A Phase III international trial presented in The Lancet in June 2010.
The study randomized whole breast radiation therapy in women with invasive ductal carcinoma who were undergoing breast-conserving surgery to intraoperative radiation therapy at the time of breast conserving surgery. This trial presented data that showed equivalent local control and survival, whether patients undergo one fraction of radiation (IORT) or seven weeks of definitive radiation therapy. From a quality-of-life standpoint for patients, this early data suggests excellent cosmetic results and a high degree of patient satisfaction and acceptance.
Careful patient selection is key to identifying the group of women that would best benefit from this technique. Selection criteria are very similar to patients who are candidates for APBI. The American Society of Breast Surgeons task force has proposed suitable patients for APBI if several criteria are met: age 45 years or older, tumor size 3 cm or less, an invasive ductal carcinoma, negative margin status at least 2 mm in all directions, and negative sentinel lymph node evaluation.
The TARGIT trial accepted women with early breast cancer if they were age 45 years or older and had undergone wide local excision for invasive ductal carcinoma. When looking at the characteristics of the tumors in the patients in the international trial, the median age was 63 years, 86 percent of the tumors were smaller than 2 cm, 90 percent expressed estrogen receptors and nodes were uninvolved in 83 percent of patients.[1]
The IORT Technique, Play-by-Play
In our experience, sentinel lymph node dissection (SLND) with frozen section diagnosis is performed at the time of the partial mastectomy and the planned IORT treatment for all invasive cancers. Noninvasive cancers have SLND at the discretion of the surgeon. Patients with positive sentinel lymph nodes on frozen section are ineligible for IORT and are treated with conventional radiation.
The patients undergo a standard partial mastectomy; however, the tissue dissection is carried down to the level of the pec fascia. Blunt dissection is performed circumferentially in order to accommodate a chest wall shield placed temporarily into the cavity for the duration of radiation to protect the chest wall from radiation damage. The diameter of the circular chest wall shielding is customized to 1 cm larger than the actual treatment balloon diameter. The goal of the temporary lead shielding is to protect the underlying heart, ribs and lung from the high dose of radiation delivered.
One of the issues with IORT is the inability to obtain margin assessment prior to delivery of the radiation. Margin assessment is performed by the pathologist in a standard fashion. In the event of a positive margin, patients are re-excised. If the re-excision is positive, patients are offered traditional radiation over five weeks, or if chemotherapy is indicated, an accelerated regimen over 16 days. In the TARGIT trial, 85 percent of patients received IORT alone and did not require additional external beam radiation.
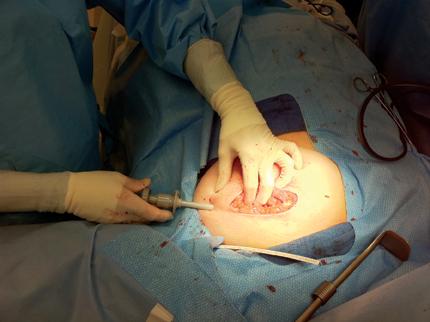
A cavity evaluation device (CED) is placed through a separate incision and filled to a desired predetermined volume, based on the partial mastectomy cavity size. Because of the intraoperative and real-time nature of the radiation delivery, the radiation plans are already pre-planned for a variety of treatment balloon sizes at 5 cc increments, based on balloon sizes from 30 cc up to 70 cc. The dose of radiation delivered is 2,000 cGy prescribed to the surface of the balloon. Once the cavity volume is determined in appropriate size using the CED, the actual treatment balloon is exchanged and placed into the cavity.
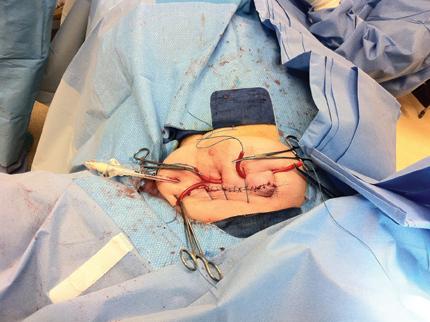
In order to temporarily close the partial mastectomy cavity, retention-type sutures are used to close the skin around the balloon. The sutures also are used to build up the subcutaneous tissue in order to increase the balloon-to-skin distance.
In order to safely deliver the radiation and reduce the risk of skin complications, the distance from the balloon surface and the skin surface should be at least 1 cm. Intraoperative ultrasound is utilized to determine this prior to initiating treatment. A second timeout is performed with the radiation and operating room teams prior to starting the radiation beam.
At this point, the radiation source is inserted into the balloon and radiation is initiated. The typical treatment time is eight to 10 minutes, depending on the balloon size. The overall treatment time of the radiation oncology team is approximately 22 minutes, and the whole IORT procedure only adds 25-30 minutes of additional total operative time.
Key personnel are in the room during the actual treatment delivery and are wearing lead aprons, thyroid shielding and conventional shielding during the radiation delivery, similar to what would be used for fluoroscopy. After radiation is delivered, the balloon is deflated and removed, the temporary shielding is removed and the surgical site is closed in a standard fashion.
Postoperatively, both the breast surgeon and radiation oncologist typically see patients at one month, six months and every six months thereafter.
Initial Challenges and After-Effects
We initially had some difficulty with the Flexi Shield Mini chest wall shielding marketed by Xoft, because the deposition of tungsten particles led to tungsten deposition seen on postoperative mammograms. This happened in two of our four patients. In the other patients, we switched to a lead-based shielding and we had no further issues with any post-mammography particles seen from the lead shielding.
The tungsten deposition was seen on post-mammographic images at two months following radiation treatment and had the particulate appearance, according to the radiologist, similar to deodorant particles on the mammogram. Neither patient required any surgical intervention, as the media and literature had reported this finding and it was not a complete surprise to our participating radiologist.
From a clinical standpoint, the amount of tungsten deposition did not interfere with future imaging, and certainly in our cases, according to the radiologist at our institution, could not be confused with malignant calcifications. The tungsten deposition covered an area just close to the chest wall, approximately 5 to 7 cm in the exact areas where the temporary shield had been placed during treatment.
The Axxent Flexi Shield Mini is an optional accessory device for IORT and is one of several shields available. The issues with the Flexi Shield eventually led to iCAD initiating a voluntary recall of the device. iCAD Inc., the parent company of Xoft, is actively addressing the situation, working closely with physician customers, impacted patients and the U.S. Food and Drug Administration (FDA). In fact, iCAD recently received clearance from the FDA for its new Axxent Rigid Shield, a stainless steel shielding device to protect tissue and/or organs from X-ray radiation.
Final Comments
IORT is emerging as an alternative to whole breast radiation therapy and outpatient APBI in a select group of women with early stage breast cancer. It is an increasingly attractive choice, offering several benefits to both patients and physicians.
From the oncologic perspective, radiation is delivered to the lumpectomy cavity as soon as the tumor is removed, before any remaining cells have a chance to reproduce. Balloon placement and tailoring of this cavity decreases the ability of geographic miss, in which the prescribed radiation dose is inaccurately delivered to the tumor bed. Moreover, in very superficially located tumors that are otherwise not amenable to APBI or without multi-channel balloon or cavity-based techniques, the use of intraoperative-placed temporary retention sutures allows the surgeon to create an adequate skin-to-balloon distance needed for safe dose delivery.
From a patient perspective, the extra 20 to 30 minutes spent by the surgeon and radiation oncologist will often negate the logistically impossible six-week or five-day requirement of whole breast radiation therapy or APBI. The use of IORT will obviate the need for mastectomy in patients who cannot travel to a radiation treatment center, which may be a significant advantage in Third World and developing countries where travel precludes patients opting for breast conservation.
When compared with other techniques, IORT is emerging as a valid patient- and physician-friendly alternative. With our initial patient experience, overall results were extremely encouraging, with patients achieving excellent cosmesis and high levels of satisfaction. As the variety of treatment options for breast cancer emerge, IORT promises excellent tumor control, cost containment and the possibility of widespread adoption.
V. Elayne Arterbery, M.D., MHSA, is currently clinical chief of radiation oncology at Crittenton Cancer Center in Rochester, Mich., and was a clinical instructor at the Johns Hopkins Oncology Center in Baltimore, Md. A graduate of Stanford University, Arterbery is well-known among her colleagues in the field of radiation oncology worldwide. For questions, contact Dr. Arterbery at (248) 844-4025 or [email protected].
References:
1 Vaidya JS, Joseph DJ, Tobias JS, Bulsara M, Wenz F, Saunders C, Alvarado M, Flyger HL, Massarut S, Eiermann W, Keshtgar M, Dewar J, Kraus-Tiefenbacher U, Sutterlin M, Esserman L, Holtveg HMR, Roncadin M, Pigorsch S, Metaxas M, Falzon M, Matthews A, Corica T, Williams NR, Baum M. “Targited intra-operative radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomized, non-inferiority trial Phase III.” The Lancet, 2010; published online June 5, 2010, pp. 1-12.



 February 18, 2026
February 18, 2026 









