Intensity modulated radiation therapy (IMRT) emerged as a new way to treat patients in the late 1990s and garnered attention because it offered the possibility to avoid radiating healthy tissue with more accuracy than conventional radiation therapy techniques. As a highly conformal 3-D therapy, its precise targeting of a tumor allows higher radiation doses to be delivered more safely. Since its inception, it has grown especially in use for cancers in the prostate, head and neck regions, as well as other areas of the body.
Its impact has been widespread. Radiation Medical Group in San Diego, Calif., says on its website that IMRT is “the single biggest improvement in radiation therapy in the last 30 years. It is very much like going from the propeller age to the jet age in aviation.” (www.rmg.md/IMRT.htm)
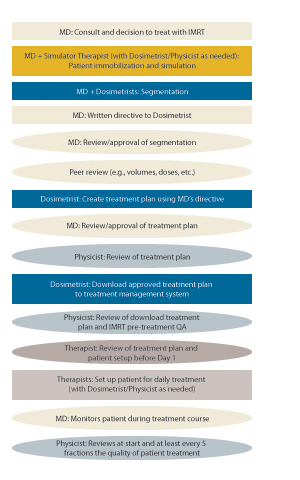
Varian, Accuray and Elekta are the primary vendors of linac technology to perform IMRT. Varian, Elekta, Philips and RaySearch offer treatment planning software, which is a critical part of the IMRT process. It is an involved and time-consuming process to create a treatment plan for IMRT and achieve the 3-D conformity. The International RadioSurgery Association (IRSA) estimates an IMRT treatment plan takes an average of two to three days per patient (www.irsa.org/imrt.html).
Because the IMRT process is complex and the doses are so high, there are more opportunities for error, so safety is a concern. In fact, the American Society for Radiation Oncology (ASTRO) kicked off a recent series of white papers addressing patient safety with “Safety Considerations for IMRT,” published in July 2011 in Practical Radiation Oncology. (www.practicalradonc.org/article/S1879-8500(11)00162-7/fulltext)
“This report provides an opportunity to broadly address safe delivery of IMRT, with a primary focus on recommendations for human error prevention and methods to reduce the occurrence of errors or machine malfunctions that can lead to catastrophic failures or errors,” the report introduction said. The document includes tools and techniques to be used by clinics to ensure the safety of their IMRT programs.
Environmental and Technical Safety Concerns
The ASTRO white paper classifies safety hazards for IMRT into two groups: environmental and technical.
Environmental concerns are not unique to IMRT, but have safety implications for many other types of treatment. They include: lack of standard operating procedures, haste (i.e., inadequate time to perform all steps in a process), habituation, incomplete understanding or misuse of procedure/equipment, an inadequate QA program and lack of continuing staff education.
The technical concerns are areas such as inadequate commissioning of the clinical IMRT program, inadequate validation of the accuracy of treatment delivery parameters, improper use of one or more parts of the planning and delivery process, and inadequate investigation of discrepancies between treatment plan parameters and QA results. The white paper presents detailed recommendations and step-by-step guidelines to facilitate a safe IMRT program. (ASTRO also is in the process of developing two more white papers as part of its series, on peer review and image-guided radiation therapy. These can have an effect on IMRT practices as well.)
Customers Adopt New Technology
According to a report from KLAS, “Radiation Therapy 2011: A Dose of New Technology,” released last November, the oncology market in general has been growing. That trend is expected to continue, thanks to the need created by an aging population, and despite the slow economy and cuts in Medicare reimbursements.
“Providers are buying, and new vendors are entering the market,” KLAS said.
The report evaluated customer satisfaction with equipment and treatment planning software used for radiation therapy, including IMRT, as well as their concerns. It noted that radiation therapy providers are anxious to have the latest technology for treatment reasons, but also as a marketing tool. “The biggest provider draw is bleeding-edge technology, not only for patients’ benefit, but also for marketing and referrals,” the report stated.
Customers also are concerned with the safety of their systems, especially as patients read negative publicity about radiation dose. They believe their systems to be safe, but look for help with safety education from their vendors.
Reliability is another key factor for customers, who want a system with minimal downtime. “Providers are really looking for reliable equipment that is technologically advanced that they are able to draw referrals on. The combination of those attributes is driving satisfaction in the oncology market,” said the report.
For additional information, visit www.KLASresearch.com.


 February 04, 2026
February 04, 2026 









