Editor’s Note: Exploring IGRT is the result of a “virtual” roundtable discussion on image-guided radiation therapy (IGRT) with notable radiation oncology experts Drs. Rod Ellis, Dan Low, Todd McNutt, George Starkschall and Charles Smith.
How would you define IGRT and what would you say are its key aspects? What is IGRT from a clinical standpoint?
Dr. George Starkschall: I think what will be key is what are you going to do with the image once you’ve captured it? There needs to some mechanism for real-time response to the particular images that you see. This could include modifying a treatment portal, or moving or realigning the patient, and being able to respond to the information provided by the image in real time or near real time.
Dr. Charles Smith: IGRT, in it’s earliest definition, existed since clinicians began using port films to visualize bony anatomy in guiding beam placement. I believe that the current general definition focuses on using imaging resources in the treatment room to ensure that highly conformal therapy (including IMRT) is delivered as planned. The two major groupings of IGRT will be associated with managing interfraction target motion (variation from day to day) and intrafraction target motion (motion of the target and regional anatomy during the treatment (i.e., respiration and/or peristalsis).
Dr. Todd McNutt: You have to identify what the different images are. Some images are anatomical, where we’re looking at geometrical changes in the patient anatomy over the course of therapy or on the day of the treatment. There are also PET images or other types of imaging devices that can be used to guide the therapy that aren’t anatomical. With these images, the information is not necessarily used to guide the geometry of the therapy but used to guide the biology of the therapy. What we should be focusing on is the anatomical changes because that is what these online imaging devices provide. When people talk about image guidance, they are typically referring to therapy devices that have imaging capabilities, and those products are primarily focused on the anatomical changes in the patient over the course of treatment or for daily patient alignment.
Dr. Rod Ellis: I agree completely, I think it is a twofold application. You can do IGRT just based on anatomy if you are looking at the anatomic images and make corrections for where that anatomy is at day to day for external beam planning.
With LDR brachytherapy, after placing the sources they are being carried with the volume from day to day, thus daily movement of the target volume is not a critical function as long as there is not significant migration of the sources. It’s a second application; that is functional imaging — whether that be SPECT or PET imaging or MRI spectroscopy that I also think of in regards to IGRT; when multimodality images are used to improve planning for radiotherapy.
If you are doing daily radiotherapy, once you’ve fused that to an anatomic image and define a treatment volume — whether it is an anatomic volume with a standard image or an anatomic volume with a functional image — you have got to be able to move day to day to track the patient’s target volume. So it is twofold, either anatomical or functional; both must be defined.
Dr. Starkschall: Are you suggesting that if we do functional imaging we must acquire daily images?
Dr. Ellis: No, if you do a treatment plan, you bring in a functional study and co-register to the anatomic study that you use to do the initial plan. Then you take that functional study that’s used at that day to plan and use it as the target that you are going to treat. You move the anatomic study from day to day and assume that the functional study moves with the anatomical study.
Dr. McNutt: This opens the question of what you actually see with the imaging modality on the treatment unit. Can you really identify what your target is from that data? You have to derive in some way that what you see in the anatomical image is a surrogate for what we’re trying to treat, which might be identified on a PET study.
Dr. Ellis: There have been several different breakthroughs that we have seen in the last year. From the software programs that allow you to do image co-registration to, more recently, hardware solutions with PET/CT or SPECT/CT that do a much better job of making sure there is no movement in the anatomy between the two studies. What is lacking today, and where the future direction is going to be, is in the portability — how do you take the image that was done in a diagnostic setting and put it into the clinic and make sure it is in the same position as the patient set-up in your therapy room or OR suite.
So, for IGRT, we need to talk about both anatomical and functional images — and fusion, whether that be software or hardware. Is fusion one of the areas where there is a serious deficit of quality technology?
Dr. Starkschall: Many methods for image registration presently exist. We can now obtain a PET image using PET/CT and easily register the PET image to the CT image. We can obtain a SPECT image on a SPECT/CT and register the SPECT image to the CT image. We will be working a lot to improve image quality of these, improving the reliability, but I think the breakthrough has occurred.
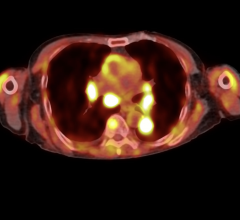
Dr. McNutt: We want to use imaging to reduce our treatment margins, because our treatment margins are derived based on set-up error and population statistics on organ motion. What anatomical image guidance allows you to do is tailor those margins to a specific patient rather than depend on population statistics. What you’ve got is the anatomical imaging for monitoring the set-up uncertainty in organ motion to determine these specific margins. When we get to the PET imaging and molecular imaging they are on a different time scale in terms of guiding the course of therapy.
With PET imaging, you might not see responses daily, but you may receive significant changes in some of the functional or molecular imaging mid-course of treatment as opposed to daily. The other thing is with some of the PET imaging there is still some uncertainty as to what you are looking at. For example, we saw some studies at ASTRO where they showed FDG studies where you had significant FDG uptake in the area where the treatment was delivered. That doesn’t mean it is diseased; that means there is a lot of sugar being taken up by the cells that have been damaged by the radiation and don’t necessarily relate to the disease.
You have to be careful with some of the biological imaging and understand what it means. I think that we are not there in terms of biological imaging, whereas I think the technology for anatomical imaging during treatment is pretty well advanced. Now we are just learning how to make it efficient enough to be used clinically.
Dr. Ellis: I think one of the biggest areas for improvement with functional imaging is how do you get a second validation of what that image shows you. If you have ever looked at a fused PET scan or a fused SPECT scan, you can adjust that contrast with the gamma levels that make the whole structure light up or none of it light up. The question is: How do you adjust it to know what reality is, where the cancer is really at? And I think in vivo techniques need to be developed to correlate with the functional studies.
Dr. Smith: Dr. Ellis has identified an important point in the use of functional imaging in treatment planning and IGRT. The determination of the target volume remains a highly subjective decision-making workspace that is the exclusive domain of the physician. Accepted values for viewing the image study have yet to be agreed upon, like imaging window and level, or what fraction of [SUV] should characterize tissue as a tumor. It is, however, agreed that the added value of functional imaging can often make the difference between curative or palliative therapeutic intent for the patient. In many types of cancer, elements of the functional and anatomic studies, often “co-registered and fused”, by an experienced clinician yield a more accurate target definition than either study might alone provide.
Dr. Starkschall: Ultimately, we need to develop some better tumor-specific contrast agents that will light up where the tumor is and nowhere else. That is still a way out.
Dr. Ellis: We have seven years’ outcome data from prostate cancer with functional imaging. The limitation of prior studies is that they rely on anatomic images. There is no way to see where the prostate cancer is; we just target the prostate. We have been using SPECT imaging to show us biological activity within that volume and targeting that for higher doses.
Do you believe that in the future we will come to a point where we will have all the tools and techniques — including IGRT — to treat all tumors in all locations in the body? Is that practical?
Dr. McNutt: If you can define the target then we have the potential of being able to treat it. Radiotherapy is traditionally a local treatment, not a systemic treatment across the body, so some patients in whom you can’t really define the target or the target extends over a large part of the body would still be difficult. But if you can define the target then it might be possible.


 December 02, 2025
December 02, 2025