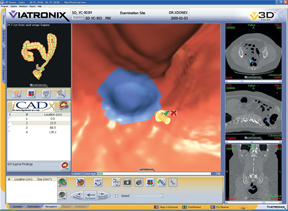
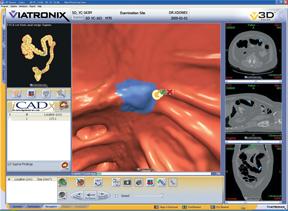
CT Colon from iCAD uses sophisticated image processing software to automatically identify polyps in CTC images.
According to the American Cancer Society, colorectal cancer is second only to lung cancer as America’s leading cancer killer each year. Over 50,000 Americans will die from colon cancer this year alone. While this is a staggering statistic, it is estimated that less than half of the Americans who are recommended to be screened for colon cancer actually get tested.
Reluctant Patients
Optical colonoscopy is the gold standard test for colorectal screening, but the procedure requires anesthesia and is not recommended or tolerated well by everyone. General reluctance to undergoing this procedure stems from the preparation, the sedation, and/or fear of the physical discomfort some may experience.
Virtual colonoscopy (VC) or CT colonography (CTC) employs cutting-edge, advanced visualization technology to produce three-dimensional images that permit a thorough and minimally invasive evaluation of the entire colorectal surface. During review of CTC exams, reading physicians “fly through” a three-dimensional view of the entire colon, as well as visualize individual two-dimensional CT slices. No sedation is required for a VC exam and patients can resume normal activity, including driving, immediately following the procedure.
In a recent Boston Globe letter, Michael Zalis, M.D., director of CT colonography at Massachusetts General Hospital and associate professor at Harvard Medical School, commented that he routinely encounters patients who have avoided screening until coming to him for CTC. He wrote, “In all these situations, patients repeatedly tell me they have been waiting for an alternative to optical colonoscopy. And generally, they’re satisfied with their experience with CTC.”
CTC continues to gain clinical validation as evidence mounts to show CTC is highly effective in the detection of clinically significant intermediate and large polyps in the colon and in that way has been proven as an effective alternative to optical colonoscopy. These studies include ACRIN’s National CT Colonography Trial1 of a screening population, which demonstrated that the accuracy of CTC is similar to optical colonoscopy. Prevention of colorectal cancer depends on detection of polyps since most cases of cancer begin with slow growing benign polyps in asymptomatic patients.
CTC Improves Performance
CMS (Center for Medicare and Medicaid Services) issued a noncoverage decision for CTC in May 2009, but the future still looks bright. Experts, including the American Cancer Society, the American College of Radiology, the American Gastroenterology Association and the United States Multi-Society Task Force on Colorectal Cancer, have published reports supporting CTC for colon cancer screening. Additionally, the large body of scientific evidence supporting CTC has satisfied and motivated numerous private insurers. Currently 26 states mandate that patients with private healthcare coverage are provided access to virtual colonoscopy.
Although VC is supported by large, multicenter clinical data, it is still possible for radiologists to overlook and miss visible polyps even with careful study review. CTC exams typically contain between 1,200 and 1,500 images per patient. With such a vast amount of data, reviewing and assimilating CTC images can be tedious and challenging to radiologists. Minimizing oversight errors will be critically important in the widespread adoption of VC as a screening tool.
CTC computer-aided detection (CAD) technology has been developed to aid radiologists by detecting and highlighting potential polyps that could be missed by a reader during their review of CTC exams. CAD has shown high standalone sensitivity in detecting polyps in CTC exams. Improvements in radiologist performance have also been shown when measured by the increased area under the receiver operating characteristic (ROC) curve and increased sensitivity, with a trade-off of lower specificity. The use of CAD has also shown trends toward improvements in radiologist performance during the interpretation of CTC exams.
These improvements in radiologist performance are possible because CAD can identify additional polyps that were not initially detected by the reader. CAD can help improve detection even if the sensitivity of the CAD system is not significantly higher than the sensitivity of the reader, since CAD may help the reader identify additional polyps not initially detected by the reader. Clinical validity of CAD is established each time CAD software is used as a second read, after initial review of a CTC exam by the reading radiologist.
Abraham Dachman, M.D., professor of radiology and director of fellowship programs at the University of Chicago, has been doing research work on virtual colonoscopy for approximately 14 years. He states that “CAD for the CTC exam can have two very important positive effects. One is [that] it’s the great equalizer. It will help make the novice reader closer to the expert reader when they’re beginning to read. And even for the well-trained moderate expert, even advanced expert, it may increase confidence of interpretation.”
“Some of the biggest advantages of using CAD are increasing sensitivity, possibly increasing specificity, and it should have a minimal effect on reading time, in our experience adding only about three minutes,” said Dachman.
Advanced Tools for CAD
CAD works in close collaboration with advanced visualization hardware and software by analyzing the original images received from CTC systems from all manufacturers. CTC CAD technology uses advanced algorithms to highlight potential polyps warranting closer review by a radiologist. This type of image analysis employs image processing, pattern recognition and artificial intelligence. CAD systems typically search for and highlight clinically significant types and sizes of polyps and adenomas, including sessile, pedunculated and flat polyps. CAD systems should be able to analyze images from patients who have used various preparation methods as well as accommodate images with either tagged or un-tagged stool.
The initial focus of CTC CAD has been on the detection and highlighting of potential polyps, but Dr. Dachman notes that “[i]n the future, CAD will incorporate various tools to help the radiologist. For example — tools to help measure polyp size, polyp volume, help compare a polyp on follow-up exams to see if it’s changed or increased in size, and possibly some cutting-edge research on polyp characterization.”
CAD for CTC is investigational in the United States, but it’s already in use throughout Europe. In May 2009 iCAD Inc., a leading provider of CAD and workflow solutions, completed a multireader, multicase CAD clinical study and has submitted for FDA 510(k) clearance of its VeraLook CTC CAD system.
As diagnostic imaging continues to progress toward safer, more cost-effective technologies that are able to find more cancers at an earlier stage, it is expected that CTC will be incorporated into many clinical practices. With the widespread addition of CAD to CTC interpretation, radiologists can focus on the areas of most concern and the areas where they have the greatest opportunity to save lives.
“Even if there were no direct reimbursement for CAD, I would still strongly recommend using CAD for CT Colonography. Most of the errors that are made, even by experts in clinical trials, are errors of observation, meaning a polyp is visible in retrospect. And that’s where CAD has its strongest application,” said Dr. Dachman.
Marc Filerman is the vice president of global marketing for iCAD Inc.
References: 1. Johnson CD, et al. “Accuracy of CT colonography for detection of large adenomas and cancers.” N Engl J Med 2008; 359:1207-17.
2. Pickhardt, et al., “Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults,” N Engl J Med 2003; 349:2191-2200.
3. Johnson CD, Harmsen WS, Wilson LA, et al. “Prospective blinded evaluation of computed tomographic colonography for screen detection of colorectal polyps.” Gastroenterology 2003; 125:311-319.
4. Cotton PB, Durkalski VL, Pineau BC, et al. “Computed tomographic colonography (virtual colonoscopy): a multicenter comparison with standard colonoscopy for detection of colorectal neoplasia.” JAMA 2004; 291:1713-1719.
5. Rockey DC, Paulson E, Niedzwiecki D, et al. “Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison.” Lancet 2005; 365:305-311.
6. Kim DH, Pickhardt PJ, Taylor, AJ “CT Colonography versus Colonoscopy for the Detection of Advanced Neoplasia,” N Engl J Med 2007; 357:1403-1412.
7. Doshi, et al., “CT Colonography: False-Negative Interpretations.” Radiology 2007;244:165-173
8. Yoshida H, Nappi J, MacEneaney P, et al. “Computer-aided diagnosis scheme for detection of polyps at CT colonography.” Radiographics 2002; 22:963-979.
9. Bogoni L, Cathier P, Dundar M, et al. “Computer-aided detection (CAD) for CT colonography: a tool to address a growing need.” Br J Radiol 2005; 78:S57-S62.
10. Taylor SA, Halligan S, Burling D, et al. “Computer-assisted reader software versus expert reviewers for polyp detection on CT colonography.” AJR 2006; 186:696-702.
11. Petrick N, Haider M, Summers RM, et al. “CT colonography with computer-aided detection as a second reader: Observer performance study.” Radiology 2008; 246:148-156.
12. Taylor SA, Aslam R, Barlow JM, et al. “CT colonography (CTC): investigation of the optimum reader paradigm using computer assisted detection (CAD) software (abstr).” In: Radiological Society of North America scientific assembly and annual meeting program 2006. Oak Brook, Ill: Radiological Society of North America, 2006; 201.


 March 03, 2026
March 03, 2026 









