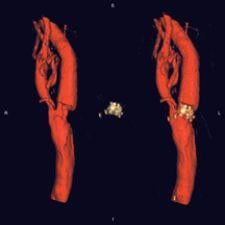
Source: Toshiba America Medical Systems
Any time a patient can be administered minimally invasive treatments percutaneously, have an increased chance at abridged recovery time and a reduced or eliminated hospital visit, it’s a step in the right direction. Efficiency is crucial, and interventional imaging is becoming more effective each year.
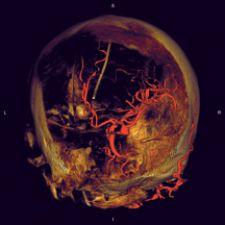
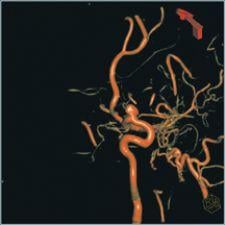
Interventional radiologists are board certified radiologists that specialize in interventions through the skin using guided imaging. Until recently, interventional imaging had been limited to maneuvering various small instruments through the complex pathways of the human body, from outside the body, guided by a 2-D fluoroscopic image. It is hard to believe that they were previously able to guide a tiny instrument through the convoluted vasculature of the human body using a 2-D fluoroscopic image, but that is what makes them such extraordinary practitioners. This, however, is no longer the case. Advanced technology is making their job easier and increasing their effectiveness. They are now able to manipulate a CT-like 3-D image on a digital flat-panel to perform these interventions, which results in significantly quicker operations that increase caseload capacity and, in many cases, can be performed in an outpatient setting.
“The interventional radiologist now has a 3-D image to navigate on,” said Karl Kellar, Americas Vascular and Intervention marketing manager, GE Healthcare. “If they’re trying to pass a catheter through the complex 3-D array of vessels, and they’re using fluoroscopy, all the vessels are superimposed over one another. [Interventional radiologists] have learned how to separate out a 2-D image into 3-D in their mind, but with a 3-D image, it is right in front of the doctor, right next to the fluoro monitor. And with control at the tableside, they can rotate that 3-D image in space, and see exactly what the passageway and the relationship of the vessels is.”
It is important to remember that these 3-D images are “CT-like” in nature. The flat-panel technology is not capable of everything a dedicated CT scanner is, but it does provide high-resolution images that significantly aide in performing complex vascular procedures.
Several manufacturers offer this CT-like image acquisition, so end-users have their choice of systems to incorporate into their interventional suites. Among these manufacturers are GE Healthcare, Philips Medical Systems, Siemens Medical Solutions and Toshiba America Medical Systems, all of which offer unique platforms.
Divergent Platforms Striving Toward a Common Goal
All of these manufacturers offer interventional radiology platforms that have the ability to acquire 3-D images for interventional procedures. It is the various specifications that set the systems apart, leaving the user the choice of which system suits them best.
GE Healthcare offers the Innova 4100, the world’s first large-format digital flat-panel X-ray system for angiographic imaging, with a number of enhancements launched at RSNA 2005.
“[GE Healthcare] actually launched a new tableside interface, which moves much more of the control of the system into the sterile environment at tableside,” said Kellar. “The other really big rollout is the maturity and protracted launch of 3-D imaging. The flat-panel detector technology has a much higher level of performance in certain types of imaging parameters, which make it much more appropriate for 3-D imaging.”
The new tableside user interface is based on speed efficiency. When the majority of control can be done at tableside, the physician makes fewer trips to the control room, and time is saved on the procedure. The Innova 4100 also has a 41- by 41-cm detector, the largest in the industry. With this larger detector, it allows the user to image the maximum amount of vascular images per dose of contrast. This is always significant because of health risks associated with contrast media. The more anatomy covered with fewer fields-of-view, the safer it is for the patient.
The Allura Xper platform from Philips Medical Systems also includes unique features on its detector, as well as other components. The system’s flat detector is equipped with 154-micron pixels for higher resolution, a complete 30-by-40-cm image area and the ability to do 2048-by-2048-pixel imaging. At RSNA 2005, Philips focused on real-time reconstruction of 3-D volumes. According to Andrew Dunn, director of marketing, Vascular X-ray, Philips Medical Solutions North America, this was significant news for Philips at the time because previously, when a rotational acquisition was done to produce a 3-D reconstruction, the reconstruction times at best were 90 seconds. Now, Philips has cut the time to five seconds, down from eight minutes just five years ago.
Siemens Medical Solutions also recently introduced enhancements for their AXIOM Artis platform. The company rolled out the second-generation of DynaCT, I-Pilot and voice controlled functionality, which is not FDA-released yet, said Mark Lothert, senior director of product marketing, Interventional Radiology, Siemens Medical Solutions.
DynaCT allows the user to acquire the CT-like 3-D image on the AXIOM Artis. These CT-like 3-D images acquired by DynaCT allow the user to visualize soft tissue objects and help avoid having to transfer the patient to an actual CT or MRI. I-pilot provides visualization and fading between the live 2-D fluoro image and its matching 3-D dataset. Lothert said it helps give a better orientation in regards to the anatomical environment, which is beneficial for image-guided procedures.
“[Voice control], which is not FDA-cleared yet, allows us to operate main functionalities of the system by a voice,” said Lothert. “The idea is that we want to give the user a chance to have a hand or fingers where they have to be, whether it be a catheter or the patient, without being forced to touch any buttons.”
On the market for about 18 months, the Infinix-i series is Toshiba’s answer to competition within the interventional imaging sphere. Its large field-of-view flat-panel detector brings uniform brightness across the image, increased resolution and high dynamic range. While the increased field-of-view is an upgrade, it is not necessarily what makes the system unique.
“One of the functions that makes the systems unique in the industry is we have the most powerful digital processor on the market,” said Raymond Dimas, product manager, Vascular Systems, Toshiba America Medical Systems. “We have the ability to do a synchronistic frame rate acquisition. What that means is, instead of just having a set for radiology, 15, 7.5, 5, 3, 2, 1 frame rates, we can vary the frame rate according to the need of the operator to maximize the image quality and to minimize the dose.”
There are other features exclusive to the Infinix-i, including: a 484-pound patient table with the addition of 220 pounds for CPR; the ability on one power PC to review both MR, CT, 2-D and 3-D images from the other major manufacturers with the 3-D workstation; and single-hand tableside control of all controls needed to operate the system geometrically, as well as other controls needed to select image and technique, according to Dimas.
With manufacturers offering the ability to see a CT-like 3-D image on a digital flat-panel detector, an extraordinary upgrade from 2-D fluoroscopy, the choice for the end-user lies in preference of various features. As solutions continue to build upon this 3-D technology, developing faster and more efficient procedures, 3-D is redefining interventional radiology and minimizing the discomfort in the word intervention.


 August 14, 2025
August 14, 2025