Kubtec has received approval to market its XPERT 40 digital specimen radiography device from the FDA. The 510(k) premarket approval notification adds to the CE, CSA, Health Canada and CDRH certifications that have already been obtained for the device.
September 5, 2007 - Agfa HealthCare, a provider of IT-enabled clinical workflow solutions and diagnostic imaging ...
September 5, 2007 - NightHawk Radiology Services, a provider of business services and clinical workflow technology to ...
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
Standard Imaging announced at AAPM the final release of RAy Film Dosimetry Software, a 3D solution for radiation ...
NovaPACS 6.6 by NovaRad offers embedded MIP (maximum intensity projection) and MPR (multiplanar reconstruction) tools to ...
September 5, 2007 - A study in the August 11 issue of The Lancet concludes that screening for breast cancer with ...
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
Siemens recently rolled out MVision, an IGRT system that is said to deliver outstanding image quality for accurate ...
Fifteen years ago, a CT scan of Nefertiti’s bust stored at Berlin’s Altes Museum, revealed that a second structure was ...
Merge Healthcare recently introduced Merge Ortho, a digital workstation solution that reportedly helps orthopaedic ...
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
September 5, 2007 - The Emergency Medical Foundation (EMF) and GE Healthcare have entered into a one-year educational ...
September 5, 2007 - Clinical software maker Allscripts said Columbia University Medical Center in New York City has ...
Toshiba has announced a complete package of post-processing 3D and 4D software solutions for MR, CT, ultrasound and ...
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
Medrad Inc. introduced at the American Healthcare Radiology Administrators (AHRA) Annual Meeting the XDS, a new extravasation detector technology for the Stellant CT Injection System that, for the first time, directly senses extravasation pooling under the skin during a CT procedure.
September 5, 2007 - The FDA has approved Evithrom (human thrombin), a blood-clotting protein used to help control ...
September 5, 2007 - Imaging Diagnostic Systems Inc. has installed a CT Laser Mammography (CTLM) system at the Tianjin ...
North American Scientific Inc. announced that its NOMOS Radiation Oncology division will introduce the recently FDA 510 ...
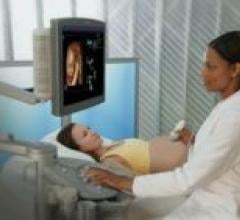
Siemens Medical Solutions Ultrasound Division recently introduced the 5.0 release of their ACUSON Antares ultrasound ...
UniWeb PACS, a multimodality Web-based solution from EBM Technologies, has received FDA clearance. EBM UniWeb can ...
September 5, 2007 — RadNet Managed Imaging Services Inc. ("RMIS"), a wholly owned RadNet subsidiary, will provide ...
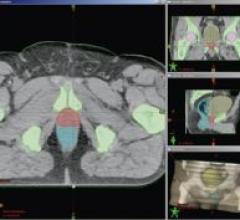
Varian Medical Systems’ new Smart Segmentation, a "smart" radiotherapy treatment planning tool, is designed to enable ...

 September 04, 2007
September 04, 2007