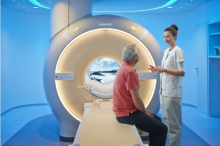
The U.S. Food and Drug Administration (FDA) announced in a new drug safety communication is this week that it is requiring a new class warning and other safety measures for all gadolinium-based contrast agents (GBCAs) used for magnetic resonance imaging (MRI). This includes increased patient education and requiring gadolinium contrast vendors to conduct additional animal and clinical studies to assess the safety of these agents.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now