Calidar's patented hardware, licensed from Duke University, that enables X-ray diffraction imaging in 4D Mammography.
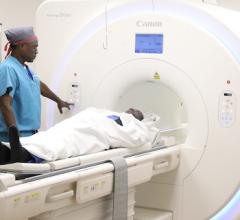
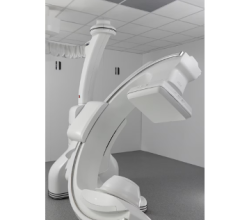
Aug. 19, 2025 — Calidar, Inc., a start-up in precision diagnostic imaging formed out of Duke University, recently announced the successful imaging of the first patient with its 4D Mammography system. The next-generation imaging system harnesses X-ray diffraction and AI to measure novel molecular-level signals of disease, offering the potential to significantly improve diagnostic accuracy. This landmark procedure was the first known in-human measurement of X-ray diffraction spectra and represents a major advancement in the field of medical imaging.
 Despite decades of innovation in medical imaging, breast cancer and other soft tissue diseases remain difficult to detect noninvasively with sufficient precision. In the United States alone, approximately 1.5 million breast biopsies are performed each year, with up to 80% of those (1.2 million) ultimately diagnosed as benign. These unnecessary procedures contribute to more than $6 billion in annual healthcare spending, a cost borne by both patients and payers. Meanwhile, delayed diagnoses due to inconclusive imaging result in an estimated 50,000 breast cancer patients annually whose treatment is postponed, increasing treatment costs and decreasing survival rates. For clinicians, unnecessary biopsies add strain to understaffed hospitals, with radiologist workloads nearly doubling and pathologist case volumes increasing by over 40% in the past decade.
Despite decades of innovation in medical imaging, breast cancer and other soft tissue diseases remain difficult to detect noninvasively with sufficient precision. In the United States alone, approximately 1.5 million breast biopsies are performed each year, with up to 80% of those (1.2 million) ultimately diagnosed as benign. These unnecessary procedures contribute to more than $6 billion in annual healthcare spending, a cost borne by both patients and payers. Meanwhile, delayed diagnoses due to inconclusive imaging result in an estimated 50,000 breast cancer patients annually whose treatment is postponed, increasing treatment costs and decreasing survival rates. For clinicians, unnecessary biopsies add strain to understaffed hospitals, with radiologist workloads nearly doubling and pathologist case volumes increasing by over 40% in the past decade.
The first-of-its-kind 4D Mammography system, designed by the Calidar team of Stefan Stryker, Josh Carpenter and Mitchell Greene, addresses these limitations by measuring how X-rays scatter at the molecular level — a process known as X-ray diffraction. This produces a unique structural signature that reflects the internal composition of breast tissue. Unlike traditional X-ray images, which rely on shape and density, X-ray diffraction imaging reveals a new dimension of diagnostic data: what the tissue is made of, not just what it looks like. In prior ex vivo studies of breast tissue, these tissue-specific fingerprints enabled classification of cancerous and benign samples with four times the precision of conventional imaging techniques.
"This is more than a study milestone — this is the start of a new era of medical imaging," said Dr. Stryker, CEO of Calidar. "X-ray diffraction has unlocked some of the most iconic achievements in science — from discovering the structure of DNA to revealing the composition of another world on the Mars rover — and now we are bringing its power into the clinic to look inside the human body in a completely new way. Our 4D Mammography system brings this capability to the challenge of breast cancer diagnostics, where high-precision, noninvasive imaging tools are urgently needed."
The first-in-human study will assess how well the 4D Mammography system can distinguish between healthy tissue and breast cancer in patients and compare performance to existing mammogram devices. The study is being conducted in collaboration with Baptist Health Hardin [BaptistHealth.com/Hardin] in Elizabethtown, KY, led by Principal Investigator Craig Kamen, MD.
"We are excited to collaborate on this next-generation research and contribute to the development of technology that could meaningfully enhance our capabilities for diagnosing breast cancer," said Dr. Kamen.
The current study focuses on the use of the 4D Mammography system in a diagnostic application, where patients already present with findings that require further evaluation. Future studies are planned to expand its use into breast cancer screening, with the goal of enabling earlier detection and improving outcomes for even more patients.
Note: The 4D Mammography system is investigational and has not been cleared or approved by the U.S. Food and Drug Administration. It is not available for commercial sale and is limited to investigational use in the United States under FDA's abbreviated IDE requirements.
For more information, please visit www.calidarmedical.com.


 March 06, 2026
March 06, 2026