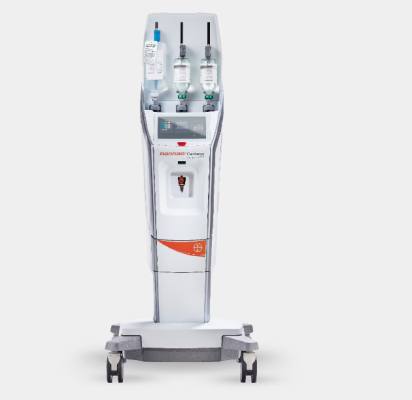
Nov. 7, 2024 — Bayer announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its MEDRAD Centargo CT injection system, a multi-patient injector that drives workflow efficiency with design features that integrate with Bayer’s portfolio of products, especially in high-volume CT suites.
Bayer’s MEDRAD Centargo CT Injection System delivers value for radiology departments as the U.S. faces a shortage of radiology technologists coupled with a rising demand for medical imaging.
“Since its launch in 2020, Centargo has served approximately 7 million patients across 49 markets world-wide,” said Sven Schmidt, Head of Region Americas Radiology at Bayer. “We know radiologists today are facing a continuously increasing workload, and the automation, integration and mobility of the MEDRAD Centargo CT Injection System helps radiology staff do more with less, allowing them more time to focus on their patients.”
MEDRAD Centargo CT injection system reduces patient set-up time, streamlines workflows and incorporates innovative technology:
- It is designed to minimize prep time as the multi patient set-up can be completed in under two minutes with the pre-assembled Day Set. The snap-in patient line auto-primes upon insertion and is ready for the next patient in less than 20 seconds.
- Streamlining data entry, the integrated barcode reader provides easy traceability and access to contrast and injection details when combined with Automated Documentation software.
- The innovative contrast injection system incorporates a piston-based technology which enables features such as Dual Flow (simultaneous delivery of contrast and saline) providing more flexibility for the most demanding protocols.
Sunnybrook Health Sciences Centre, Toronto, Canada, was the first hospital in North America to install and integrate Bayer’s MEDRAD Centargo CT Injection System in both acute care and outpatient settings.
“We've found the ease of using the Centargo injection system is having a real impact on our technologists' ability to do their work quickly and with less worry," says Mike Minoo, Manager, CT and Interventional Radiology at Sunnybrook. "For example, because I am spending less time preparing the injector and its components, I have more time to spend with the patient, increasing their comfort level and understanding of the procedure. The quality of this interaction provides a better overall experience for both myself as a clinician and the patient."
With the rising demand for imaging, radiology technologists are expected to work faster and more effectively while maintaining the highest safety standards. Bayer’s technology advancements and innovative design address these needs while incorporating more automation and workflow efficiencies. The MEDRAD Centargo CT injection system was designed with two check valves in the patient line to mitigate cross-contamination and inlet and outlet air detection with air removal technology within the day set.
The MEDRAD Centargo CT injection system enables radiology technologists to focus more on patient care and reduces process and administrative burdens for healthcare providers, specifically in high throughput settings. By integrating with Bayer’s portfolio of imaging and workflow solutions, healthcare providers can optimize contrast-usage and enable connectivity to the modality worklist and scanner.
The MEDRAD Centargo CT injection system’s innovative and user-friendly functionality was awarded with the Red Dot Design Award 2020. In addition, AMCOR, a leading company in responsible packaging awarded the AmeriStar Award for the Quick Pick & Carry Hospital Box-Style Pouch. The innovative packaging solution sets a new standard in medical device packaging by seamlessly integrating functionality, sustainability, and cost-effectiveness.
For more information about MEDRAD Centargo, visit www.radiologysolutions.bayer.com.


 March 04, 2026
March 04, 2026 









