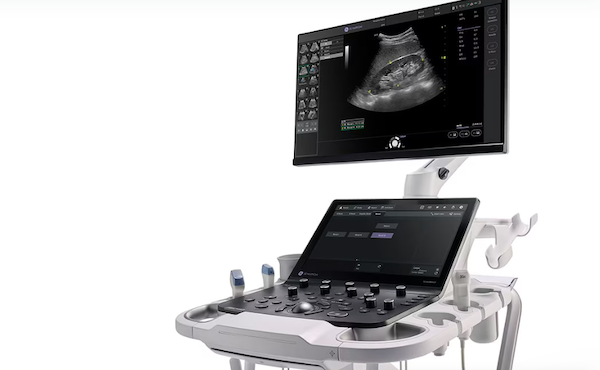
Oct. 14, 2024 — On Oct. 14, GE HealthCare launched Versana Premier, the latest release within the Versana ultrasound family of reliable, affordable, easy-to-use, and versatile ultrasound systems.
Versana Premier offers automation and AI-enabled productivity tools to improve workflow and clinical features designed to enhance clinical efficiency and accuracy, while delivering an effortless user experience and helping clinicians best meet their patient needs.[i] The Versana Premier is designed to meet the multi-purpose needs of healthcare professionals across diverse clinical specialties and care areas, including general practice, OBGYN, musculoskeletal (MSK), and cardiology.
“The rising demand for imaging has driven the increasing use of ultrasound in primary care. Ultrasound is emerging as a key tool in these clinical settings, enabling accelerated diagnoses and improving patient outcomes and satisfaction,” said Phil Rackliffe, president and CEO of Ultrasound and Image Guided Therapies, GE HealthCare. “Versana Premier is equipped with advanced automated workflow and productivity tools, including educational resources, that can support these clinicians who are often the frontline of disease identification and diagnosis for their patients.”
With advanced features that are intuitive and innovative, Versana Premier empowers clinicians to deliver high-quality care and accurately make decisions to reach a diagnosis with the greatest of ease. Powered by VisionBoost Architecture and accompanied by high-quality probes, including XDclear technology, first-class display technology and new imaging engine, Versana Premier provides exceptional image quality with faster speed.[ii] Users can also optimize images while scanning with Whizz, which continuously optimizes imaging in real time, and adjust color display with Whizz Color Flow. Other features to support clinicians in clinical decisions include OBGYN imaging tools, like Whizz RenderLive and V-Live 2.0, to capture 3D/4D imaging to clearly follow the baby’s development.
With its enhanced ergonomics, automated tools, service and support, the latest release of Versana Premier offers advanced features for simplified diagnostics and guaranteed time savings.[iii] Clinicians can experience up to a 38% faster workflow with Whizz clinical features available on the system.[iii] Furthermore, AI-powered Whizz LabeI can automate labeling of the right kidney, gallbladder and liver, and the new Whizz Follicle can assist with volume calculations and measurements.
“Versana Premier is remarkably user-friendly and image quality in B-Mode is easy to optimize, making it possible to create amazing images," said Dr. Martin Altersberger, Director of Echo Laboratory, State Hospital Steyr, Austria. "The ultrasound system integrates advanced functions with optimized image quality and educational resources, which can be used in various settings. It can, therefore, adequately support clinicians in a wide range of assessments and diagnoses.”
Versana Premier is the highest-tier ultrasound system in the budget-friendly Versana ultrasound family, powered with smart clinical features and streamlined workflow tools to enhance efficiency and provide optimal care to a wide range of patients every day.
In the United States, Versana Premier is 510(k) cleared by the U.S. Food and Drug Administration and is currently commercially available, with shipments beginning later this month.
For more information on Versana Premier, visit: https://www.gehealthcare.com/products/ultrasound/versana/versana-premier.
[i] Select features may not be available in all regions
[ii] Compared to Versana Premier R2
[iii] GE HealthCare-sponsored usability study, using Whizz. Study was conducted in December 2022. JB24263XX


 March 02, 2026
March 02, 2026 









