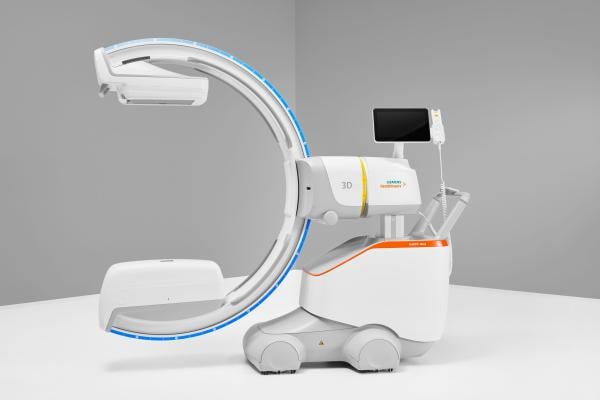
Siemens Healthineers has announced the Food and Drug Administration (FDA) clearance of the CIARTIC Move, a mobile C-arm with self-driving capabilities. Image courtesy: Siemens Healthineers
March 21, 2024 — Siemens Healthineers has announced the Food and Drug Administration (FDA) clearance of the CIARTIC Move, a mobile C-arm with self-driving capabilities. The system accelerates and standardizes 2D fluoroscopic and 3D cone-beam computed tomography (CT) imaging for surgeons and operating room teams in hospitals and outpatient facilities, bringing consistency to automated workflows and reducing imaging time during operations. Designed to address the needs of orthopedic, trauma, and spine surgery, the CIARTIC Move also can be used in thoracic, vascular, cardiovascular, and general surgery, as well as urology and interventional pulmonology.
“With the FDA clearance of the CIARTIC Move, Siemens Healthineers proudly introduces our first self-driving mobile C-arm, which can provide much-needed relief for overtaxed operating room teams by automating and accelerating intraoperative imaging workflows to a previously unseen degree,” said April Grandominico, vice president for surgical therapies in the Advanced Therapies business at Siemens Healthineers North America.
The CIARTIC Move has the potential to address challenges related to intraoperative imaging caused by staff shortages and overloaded surgical teams in the operating room (OR). During many OR procedures, the C-arm must be repositioned frequently to give surgeons the exact anatomical views they need. With conventional mobile C-arms, this repeated manual repositioning can be stressful, time-consuming, and prone to error. The CIARTIC Move is fully motorized from the C-arm down to its wheels, with self-driving capabilities to automate imaging workflows and drive consistency. These automated workflows reduce the time, effort, and workforce capacity required to manually move and position the C-arm. Up to 12 procedure-specific positions can be stored along with related imaging parameters. During a procedure, the stored positions and imaging parameters can be recalled at the touch of a button, without prolonged discussion between the surgeon and OR team. This level of automation helps the surgeon and OR staff easily reproduce images at the desired angle or return the C-arm to park and table positions. Preclinical testing shows significant intraoperative time savings—of almost 50% during spine surgery, for example, and 55% in pelvic surgery, over traditional mobile C-arms.¹ A single user can fully operate the system remotely via a wireless control, even from within the sterile field.
The system can be moved effortlessly due to its fully motorized chassis and touch-sense handles. Active sensing technology protects against collision by scanning for obstacles while in transport mode and stopping all motorized movement if an obstacle is detected.
¹ Data on file
For more information: www.siemens-healthineers.com


 March 06, 2026
March 06, 2026 









