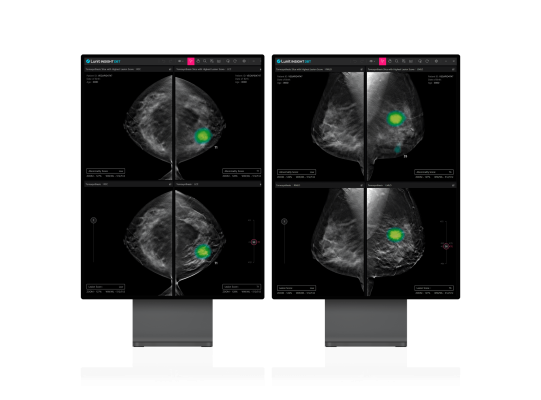
November 22, 2023 — Lunit, a leading provider of AI-powered solutions for cancer diagnostics and therapeutics, today announced that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its 3D Breast Tomosynthesis (DBT) AI solution, Lunit INSIGHT DBT. This approval signifies a major step forward in the fight against breast cancer, enabling Lunit to make a full-fledged entry into the US breast screening market.
Lunit INSIGHT DBT is an AI algorithm engineered to analyze 3D images generated by DBT equipment. DBT imaging offers a faster and more accurate breast cancer diagnosis compared to traditional 2D mammography screenings.
With DBT's capability to provide more precise breast cancer examinations, the demand for this technology is high, especially among advanced medical institutions in the US, which accounts for over 64% of the global demand for DBT. As of November 2023, 88% of the total 8,850 facilities in the US certified by the Mammography Quality Standards Act and Program (MQSA) are certified units with DBT.[1]
"The US is the biggest player in the global breast screening market, accounting for up to 40% of the market share. More than 40 million mammography screenings are reported in the US annually," said Brandon Suh, CEO of Lunit. "Given this substantial market influence, achieving FDA clearance for Lunit INSIGHT DBT not only solidifies our presence in the largest market but also marks a significant milestone in our mission to revolutionize breast cancer diagnosis and, ultimately, save more lives."
With this latest FDA clearance, Lunit continues its journey to bring groundbreaking AI breast imaging to the forefront of cancer diagnosis, promising a future where early detection and treatment of breast cancer becomes more accessible and accurate.
This FDA clearance follows Lunit's previous achievements in November 2021, when it received FDA clearance for its chest X-ray triage solution 'Lunit INSIGHT CXR Triage' and for its AI-powered mammography analysis solution 'Lunit INSIGHT MMG.' In addition, Lunit INSIGHT DBT has been cleared for Europe by being CE-marked under Europe's latest Medical Device Regulation (EU MDR) since March 2023.
For more information: https://www.lunit.io/en
Reference:
[1] MQSA National Statistics: https://www.fda.gov/radiation-emitting-products/mqsa-insights/mqsa-national-statistics


 March 10, 2026
March 10, 2026 









