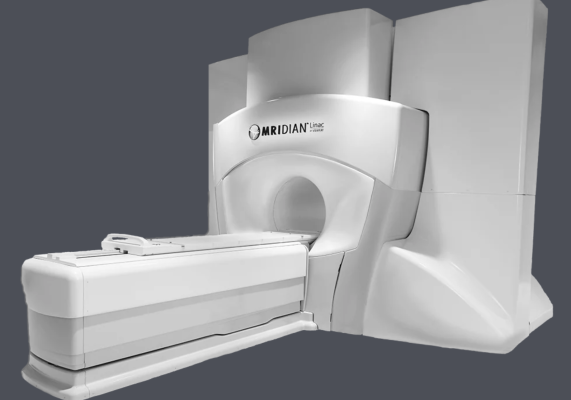
November 2, 2022 — ViewRay, Inc. announced the launch of a phase III randomized controlled trial titled "Locally Advanced Pancreatic cancer treated with ABLATivE stereotactic MRI-guided adaptive radiation therapy" – also known as LAP-ABLATE. LAP-ABLATE will compare stand-alone multi-agent chemotherapy, which is the current standard of care for patients with locally advanced pancreatic cancer, to patients receiving a combination of chemotherapy and 5-fraction MRIdian SMART (stereotactic MR-guided adaptive radiotherapy). The study is designed to demonstrate superior overall survival in patients receiving post-chemotherapy MRIdian SMART. The anticipated enrollment target is 267 patients (NCT05585554).
Although surgery is considered a potentially curative treatment for non-metastatic pancreatic cancer, less than 20 percent of patients are candidates. Of the remaining patients, approximately 40 percent have locally advanced pancreatic cancer (LAPC), and another 40 percent have distant metastases at diagnosis. LAPC is usually not resectable because the tumor encases major abdominal blood vessels.
"This first-of-its-kind prospective multi-center international randomized controlled trial was prompted by the exciting results of several recently published ablative MRIdian SMART studies that demonstrated markedly improved treatment efficacy and reduced toxicity compared to outcomes from non-ablative radiation therapy," said Michael D. Chuong, M.D., lead investigator of the LAP-ABLATE trial and Medical Director of Radiation Oncology at the Miami Cancer Institute, part of Baptist Health South Florida. "While prior studies of chemotherapy plus non-ablative radiation have not shown an improvement over chemotherapy alone for patients with LAPC, we believe MRIdian's advanced capabilities overcome the many limitations of other radiation treatment modalities. Ablative doses with MRIdian SMART can provide pancreatic cancer patients a clinically meaningful improvement in long-term survival while maintaining an excellent quality of life and rarely causing significant side effects."
Conventional chemotherapy plus non-ablative radiotherapy has not demonstrated improvements to overall survival. Further studies have supported the notion that significantly escalating the radiation dose to an ablative range may improve overall survival. But the feasibility of delivering ablative radiation dose to the pancreas has historically been limited. Previous clinical trials have resulted in a high risk of injury to the stomach and nearby bowel loops, resulting in severe side effects such as pain, bleeding, or obstruction.
"Pancreatic cancer is one of the most challenging diseases to treat, and while currently available therapies can have a meaningful impact for a proportion of patients, they are non-curative for the vast majority," said Martin Fuss, M.D., Chief Medical Officer at ViewRay. "LAP-ABLATE will evaluate the combination of chemotherapy and MRI-guided ablative dose radiation therapy to determine MRIdian's role in improving overall survival outcomes and expanding the treatment options for patients with locally advanced disease."
MRIdian integrates MRI technology, radiation delivery, and proprietary software to locate, target, and track soft-tissue and tumors. By providing real-time continuous tracking of the tumor and organs-at-risk, MRIdian enables automatic gating of the radiation, turning the beam off if the target moves outside user-defined margins. This allows for precise delivery of the prescribed dose to the target while sparing surrounding healthy tissue and critical structures.
"Despite major advances in other oncology indications, treatment options for pancreatic cancer remain severely limited, including for patients with locally advanced disease," said Julie Fleshman, President and CEO of the Pancreatic Cancer Action Network (PanCAN). "At PanCAN we support patient involvement in clinical trials as a way to gain access to some of the best treatment options and most cutting-edge approaches. We're looking forward to the results of the LAP-ABLATE trial and the opportunity to validate a potential breakthrough therapy for this group of patients."
For more information: https://viewray.com/mridian-treatment-centers/
Find more ASTRO22 content here
Related Content:
Varian Receives Investigational Device Exemption (IDE) for Flash Technology Clinical Trial, FAST-02
VIDEO: First FLASH Proton Therapy Trial Completed in Humans
MRIdian Clinical Studies and Initial MRIdian A3i Clinical Experience to be Highlighted at ASTRO 2022


 February 13, 2026
February 13, 2026 









