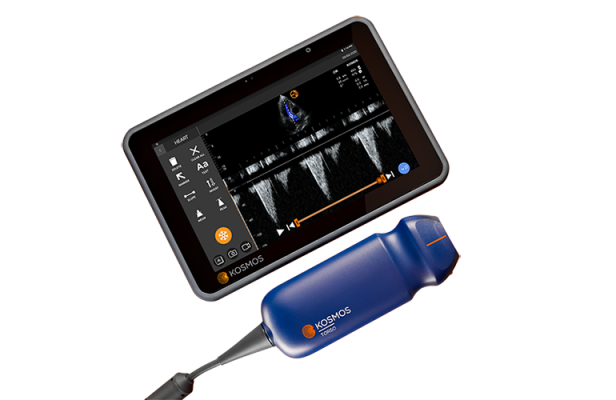
August 26, 2022 — EchoNous, the leader in ultra-portable, AI-guided point-of-care ultrasound (POCUS) tools and software, today announced an alliance with electronics giant Samsung, makers of the Galaxy line of mobile tablets. Kosmos, EchoNous’s powerful AI-guided handheld ultrasound tool, will now be able to run on off-the-shelf Galaxy Tab Active Pro tablets; models 12.0 and higher.
Made possible through the EchoNous/Samsung alliance, this new compatibility will lower the overall price point of the Kosmos platform, which already costs tens of thousands of dollars less than the cart-based models that Kosmos has been benchmarked against. This compatibility is only possible because of the Active Pro tablet’s exceptional speed, power, and battery life.
“Even for the largest medical providers in the world, every dollar saved is one that can be spent saving another life, and this alliance with Samsung makes it possible for our platform to run on a more economical, off-the-shelf tablet,” explained Kevin Goodwin, CEO of EchoNous. “Doctors can now use a tablet that they may already be familiar with and it will still run our platform flawlessly because of its inherent power.”
Until now, the Kosmos platform has only been operable with Kosmos Bridge, EchoNous’s custom-designed display. Kosmos Bridge was designed specifically for medical use and features minimal surface area and system buttons that are protected in sealed silicone rubber. While the product will remain on the market, compatibility with Galaxy Tab Active Pro models creates more options for medical practitioners and hospital procurement staff.
Kosmos is the only handheld ultra-mobile tool that offers diagnostic-grade imaging with continuous-wave Doppler capability. Kosmos’ AI-driven system enables automated assessment of systolic heart function at bedside and is the only device in its category to provide diagnostic-quality scans while also meeting HIPAA requirements for data collection, storage, and transmission. Kosmos is rapidly emerging as a tool of choice for cardiologists and critical care medicine.
“Our overarching goal is to democratize the use of ultrasound in bedside medicine. When we can utilize existing technology for that purpose, we make these tools that much more accessible,” added Goodwin. “And Samsung customers - even if they’re not medical personnel or never need an ultrasound - can feel good knowing that the devices they depend on for work and play are also helping diagnose patients and save lives around the world.”
For more information: http://www.echonous.com


 February 13, 2026
February 13, 2026 









