November 2, 2021 — Prelude Corporation, a leader in molecular diagnostics and precision medicine for early-stage breast cancer, presented data in an oral scientific session presentation at the American Society for Radiation Oncology (ASTRO) 63rd Annual Meeting held Oct.24 – 27, 2021, at McCormick Place West in Chicago, IL. Data presented demonstrates use of DCISionRT and its response subtype (Rst) was able to identify women with ductal carcinoma in situ (DCIS) (Stage 0 breast cancer) who remain at an elevated risk of recurrence despite receiving breast conserving surgery (BCS) and radiation therapy (RT).
ASTRO Presentation #513, titled Biosignatures to Optimize Adjuvant Radiation Therapy Use in Patients with DCIS with High Risk Clinicopathologic Features examined 485 women diagnosed with DCIS and treated with BCS, with or without RT. The study examined the utility of DCISionRT and its response subtype by classifying these women into three risk groups: Low Risk—those who could safely omit RT, Elevated Risk with Good Rst—those who benefited significantly from RT, and Elevated Risk with Poor Rst—those with increased recurrence rates post-treatment with BCS and RT who may need alternative therapeutic strategies beyond standard treatment.
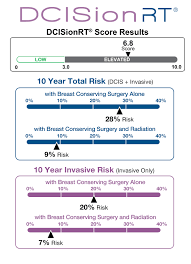
“Women classified into the Elevated Risk group with Good Rst by DCISionRT had significant benefit from adjuvant radiation, with 10-year risk reduced to 5%,” said Frank Vicini, M.D., Radiation Oncologist at GenesisCare, member of NRG Oncology, and presenter of the study. “However, patients classified into the Elevated Risk group with a Poor Rst had a 25% risk of recurrence at 10 years despite being treated with both BCS and adjuvant RT.”
“For the first time, this study demonstrates the ability to identify patients who have high recurrence risk after BCS and RT,” said study investigator Chirag Shah, M.D., Director of Breast Radiation Oncology, Department of Radiation Oncology, Taussig Cancer Institute, Cleveland Clinic, Cleveland, OH. “This additional information is critical and will help guide treatment decisions, since Elevated Risk with Poor Rst patients may benefit from supplemental therapeutic strategies.”
“Our ongoing clinical studies reinforce the medical need for personalized treatment decisions for women upon a DCIS diagnosis,” says Dan Forche, President and CEO of PreludeDx. “Our widely accessible DCISionRT test gives physicians and their patients the utmost confidence in their DCIS treatment decisions. As we continue the development of our precision testing pipeline, including our response subtype biosignature, we look forward to delivering predictive tools that positively impact treatment decisions and provide peace of mind to even more patients with early-stage breast cancer.”
For more information: www.preludedx.com


 February 06, 2026
February 06, 2026 









