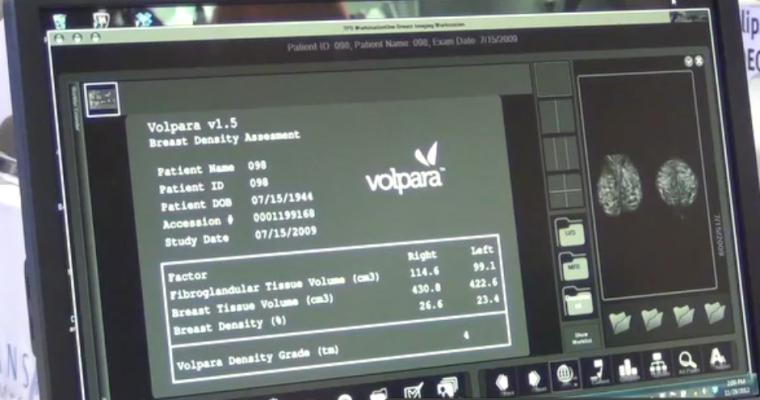
November 29, 2017 — The importance of quantitative analysis in breast imaging was the focus of numerous abstracts accepted for presentation at the 103rd Annual Radiological Society of North America (RSNA) meeting, Nov. 26-Dec. 1 in Chicago. Four abstracts highlighted the use of Volpara Solutions' quantitative analysis tools for breast imaging. The tools provide actionable quantitative metrics for clinicians, including volumetric density, personalized dose, potential high risk, and other factors designed to maintain accuracy and drive consistent quality in breast screening.
Two research groups used Volpara to examine screening performance across breast density categories. In the study, "The Relationship between Quantitative Breast Density, Age and Cancer Detection Rate in a Large UK Breast Screening Population," Louise Wilkinson and colleagues looked at the influence of age and density on the cancer detection rate (CDR) in the UK screening program. Researchers included 70,435 screening mammograms from 63,577 women with 557 breast cancers, for an overall CDR of 0.8 percent. Breast density was assessed and categorized by Volpara Density Grades (VDG). The cohort was divided into nine age groups to compare CDR in the lowest (VDG 1) and highest (VDG 4) density women, stratified by age. Results demonstrated that in younger women, extreme density (Volpara VDG4) appears to correspond to higher CDR, while the opposite is true for older women. The results suggest that the relationship between cancer detection rate and density may vary with age and thus age should be taken into account when stratifying women into different screening strategies based on density.
Additionally, results from the UK TOMMY trial established that screening sensitivity appears to be significantly increased for women with intermediate density (4.5 to 15.5 percent Volpara density) compared to those with very high or very low density. In the study, "Using Quantitative Analysis to Understand How Performance of Digital Breast Tomosynthesis Varies with Breast Density," the addition of digital breast tomosynthesis (DBT) significantly increased the specificity of 2-D mammography for all breast density subgroups but only significantly increased sensitivity in patients with quantitative breast density in the middle ranges. The results suggest that DBT may not improve cancer detection in women with very low or very high breast density.
In "Radiation Dose from Tomosynthesis and Digital Mammography versus Quantitative Breast Density," VolparaDensity was used to measure personalized radiation dose across VDGs on 4,764 DBT exams and digital mammograms, obtained by the same acquisition system in the same breast compression. Results showed that the mean glandular dose (MGD) for the four breast density categories were higher with tomosynthesis than mammography. However, the results also show an inverse relationship between VDG and dose in the DBT exams, and that the relative dose increase is higher for fatty breasts and lower for dense breasts.
Melissa Hill, Ph.D., of Volpara presented results about a new contrast-to-noise ratio (CNR) metric in "A Contrast-to-Noise Ratio for Clinical Mammographic Images." The novel approach for objectively measuring CNR on clinical images (as opposed to phantom) shows near-perfect correlation to EUREF's current method of measuring CNR from phantom images. As the first description of an objective CNR measure that can be made directly from mammographic images, this technique may enable real-time and patient-specific image quality evaluation.
Volpara Solutions showcased its entire suite of quantitative breast imaging tools, including introducing near real-time positioning feedback, during RSNA.
For more information: www.volparasolutions.com


 February 23, 2026
February 23, 2026 









