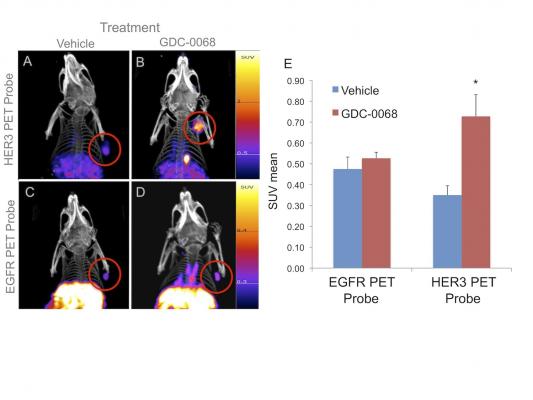
After molecularly targeted therapy with an AKT inhibitor, the receptor tyrosine kinase (RTK) HER3 increases in this particular tumor, but the RTK EGFR does not. In this example, starting HER3 inhibitor therapy in addition to AKT inhibitor therapy is likely to be beneficial, but the addition of EGFR inhibitor therapy is not likely to be beneficial for treating this tumor. Image courtesy of Massachusetts General Hospital, Boston.
September 20, 2016 — Cancer biologists know that inhibitor-mediated feedback loop changes (increased expression of a cell surface receptor in response to target inhibition) can result in breast cancer treatment failure and the need for additional therapy. A recent study by researchers at Massachusetts General Hospital in Boston and at Memorial Sloan Kettering in New York City shows that imaging of these cell surface receptor changes with positron emission tomography (PET) probes specific to epidermal growth factor receptor 1 (EGFR) and human epidermal growth factor receptor 3 (HER3) directly addresses this unmet need in cancer therapy decision-making, while avoiding the need for repeated biopsies. The study is reported in the September issue of The Journal of Nuclear Medicine.
“In some cases, cancer cells can adapt quickly to overcome these pathway blocks by increasing the upstream signaling receptors that help drive these pathways — analogous to cancer cells turning a faucet a few turns to increase the water pressure through a hose to overcome a kink we placed in the hose downstream,” explains Umar Mahmood, M.D., Ph.D., of the Athinoula A. Martinos Center for Biomedical Imaging at Massachusetts General Hospital in Boston.
“We can image that upregulation of cell surface signaling receptors and, in fact, can image which specific surface receptors have increased expression after downstream targeted inhibitor therapy is started,” he adds. “This allows the rational addition of a second therapy to block this inhibitor-mediated signaling receptor increase. In a game of chess with the cancer cell, we have made a move by blocking a signaling node. The cancer cell has responded by increasing a cell surface receptor to overcome this block, and our imaging allows us to make an optimal move to specifically block the type of cell surface receptor the cell increased.”
The imaging studies, using newly developed PET probes specific to EGFR and HER3, show that changes in receptor tyrosine kinases (RTK) expression indicative of resistance to phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) inhibitors can be seen within days of therapy initiation, and are of sufficient magnitude to be useful for clinical interpretation as this technology is translated. Noninvasive PET monitoring of these RTK feedback loops should help to rapidly assess resistance to PI3K and AKT inhibitors and guide selection of an appropriate, additional therapeutic regimen for the individual patient.
Authors of the article “Differential Receptor Tyrosine Kinase PET Imaging for Therapeutic Guidance” include Eric Wehrenberg-Klee, N. Selcan Turker, Pedram Heidari, Benjamin Larimer, Dejan Juric, and Umar Mahmood, Massachusetts General Hospital, Boston, Massachusetts; José Baselga and Maurizio Scaltriti, Human Oncology and Pathogenesis Program, Memorial Sloan Kettering Cancer Center, New York, New York.
This research was supported by NIH U01CA084301, P50CA127003, and U01CA143056; by a “Stand Up to Cancer” Dream Team Translational Research Grant, a Program of the Entertainment Industry Foundation (SU2C-AACRDT0209) and the Breast Cancer Research Foundation; and by the Dubai Harvard Foundation for Medical Research (DHFMR, grant number 223439).
For more information: www.jnm.snmjournals.org


 February 17, 2026
February 17, 2026 









