May 17, 2016 — Advanced breast ultrasound technology provider Delphinus Medical Technologies Inc. announced it is relocating its corporate headquarters from Plymouth Township, Mich., to Novi, Mich. in mid-May.
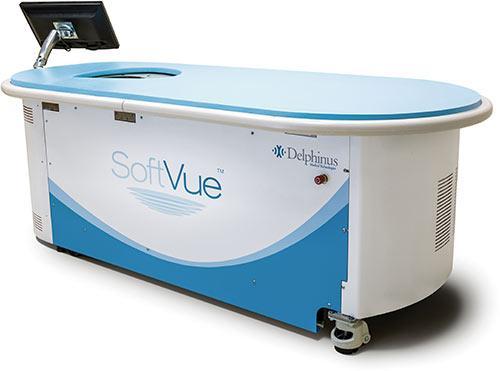
The new headquarters is nearly triple the size of its prior location, growing from 7,500 to 21,000 square feet. This will allow Delphinus to expand its staff, dedicate more space to research and development, and prepare for commercialization of SoftVue, a whole breast ultrasound system that allows physicians to image the entire breast for diagnostic imaging purposes.
The expansion is the latest in a recent series of achievements for the company. In December 2015, on the heels of a Series C venture round of $40 million, Delphinus added two new senior-level advisors to its board of directors, as well as a new vice president of quality and regulatory affairs.
One of the additions to the new facility will be a dedicated demonstration room that will allow the company to showcase SoftVue and image review to site visitors.
As an ultrasound technology, SoftVue offers the potential to not only assist physicians in finding more cancers, but also to reduce the false-positive rates that have troubled other breast imaging techniques. SoftVue’s design incorporates a circular ultrasound transducer featuring proprietary TriAD (triple acoustic detection) technology. This multi-dimensional advancement presents cross-sectional ultrasound slices through the entire volume of breast tissue, capturing not only reflected echoes in a 360-degree array, but also signals passing through the breast, depicting tissue characterization.
SoftVue is indicated for use as a B-mode ultrasonic imaging system and is not intended to be used as a replacement for screening mammography.
For more information: www.delphinusmt.com


 February 18, 2026
February 18, 2026 









