January 8, 2015 — Supplemental ultrasound screening for all U.S. women with dense breasts would substantially increase healthcare costs with little improvement in overall health, according to senior author Anna Tosteson, ScD, at Dartmouth Hitchcock's Norris Cotton Cancer Center and The Dartmouth Institute for Health Policy and Clinical Practice.
In a study released in the Annals of Internal Medicine, Tosteson and colleagues, including lead author Brian Sprague, M.D., provide evidence on the benefits and harms of adding ultrasound to breast cancer screening for women who have had a negative mammogram and also have dense breasts. The study will help inform the national legislative discussion about potential regulations requiring health providers to tell women if their mammogram shows that they have dense breasts.
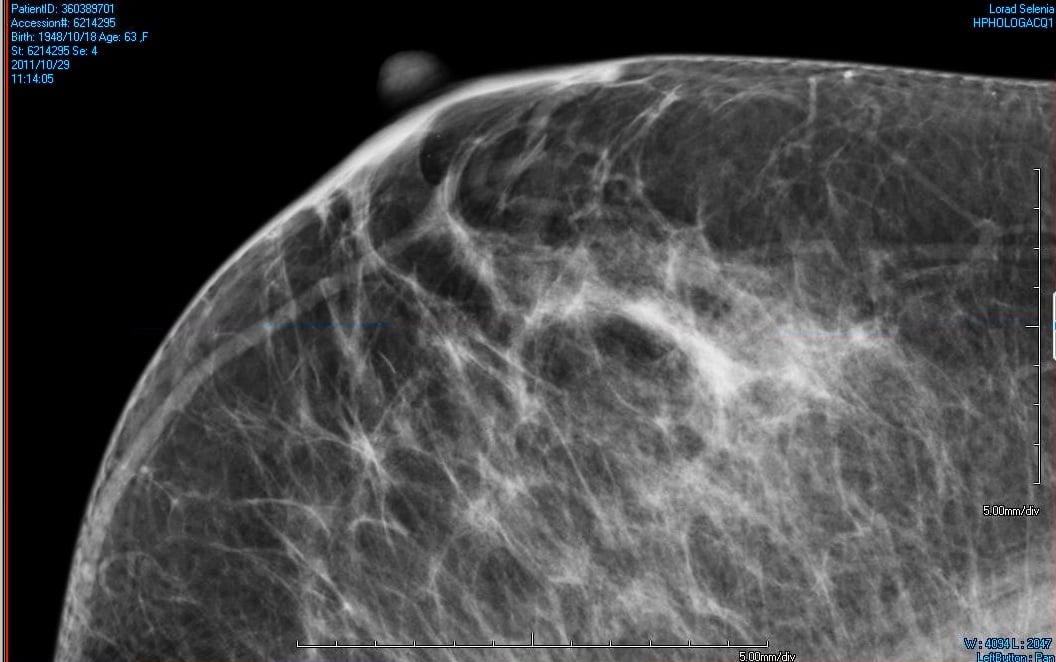
Dense breasts are a risk factor for breast cancer and also make it more difficult to recognize potential problem areas amongst the dense tissue on screening mammograms. Tosteson explained the impact of the research. “Our study is timely because with existing breast density notification laws in some 19 states, and with national legislation pending, it is critical that we understand what approaches to supplemental breast cancer screening are most effective for women with dense breasts.”
The study estimates that, for every 10,000 women between the ages of 50-74 with dense breasts who receive supplemental ultrasound screening after a normal mammogram, about four breast cancer deaths would be prevented, but an extra 3,500 biopsies would be given to women who did not have breast cancer.
Tosteson and colleagues used data from the Breast Cancer Surveillance Consortium (BCSC) and three simulation models developed independently within the National Cancer Institute (NCI)-funded Cancer Intervention and Surveillance Modeling Network consortium to evaluate the health outcomes and expense of supplemental screening via ultrasound. Because 40 percent of women from the United States who are 40 to 74 years old are estimated to have dense breasts, the value of notifying them of their status and recommending next steps in screening for breast cancer is of national significance.
Tosteson and colleagues recently published a separate simulation modeling study using preliminary data on digital breast tomosynthesis that suggested the new technology may provide an effective way to screen women with dense breasts. Tosteson cautioned that, “Those projections were based on very limited data from U.S. populations and we are expanding these data through our ongoing NCI-sponsored research within the Breast Cancer Surveillance Consortium and the PROSPR (Population-based Research Optimizing Screening through Personalized Regimens) Consortium.”
Funding for this study was provided by the National Cancer Institute–funded BCSC and the National Cancer Institute. The collection of BCSC cancer and vital status data used in this study was supported in part by several state public health departments and cancer registries throughout the United States.
Study authors were Brian L. Sprague, Ph.D.; Natasha K. Stout, Ph.D.; Clyde Schechter, M.D., MA; Nicolien T. van Ravesteyn, Ph.D.; Mucahit Cevik, MS; Oguzhan Alagoz, Ph.D.; Christoph I. Lee, M.D., MSHS; Jeroen J. van den Broek, MS; Diana L. Miglioretti, Ph.D.; Jeanne S. Mandelblatt, M.D., MPH; Harry J. de Koning, M.D., Ph.D.; Karla Kerlikowske, M.D., MS; Constance D. Lehman, MD, Ph.D.; and Anna N. A. Tosteson, ScD.
For more information: www.breastscreening.cancer.gov


 February 17, 2026
February 17, 2026 









