September 8, 2014 — Mevion Medical Systems announced that MedStar Georgetown University Hospital has received final approval to begin construction of the first proton therapy center in Washington, D.C., at its Lombardi Comprehensive Cancer Center.
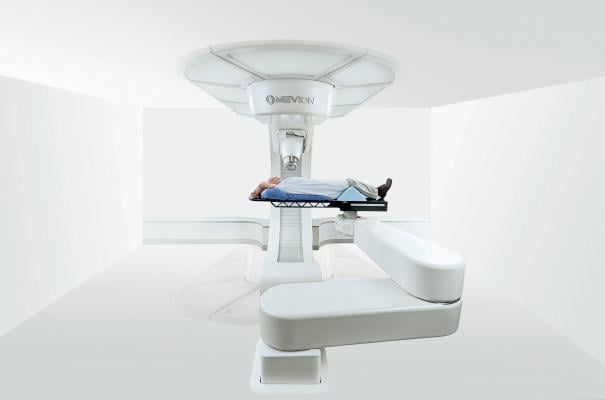
Georgetown Lombardi is the only cancer center in the D.C. area to earn the National Cancer Institute (NCI) designation as a comprehensive cancer center. The Mevion S250, the world’s smallest single-room proton therapy system, delivers the same precise, noninvasive treatment advantages and capabilities of larger, more expensive proton therapy systems but with a significantly reduced footprint, improved reliability, more efficient patient access, and dramatically lower implementation and operational costs.
Proton therapy is an advanced form of external radiation treatment that aims a powerful beam of protons at a tumor with incredible accuracy. Because of the special properties of protons, treatments result in less damage to surrounding healthy tissue and a reduced risk of secondary malignancies. Proton therapy is commonly used to treat a wide range of cancers in sensitive locations, such as the central nervous system, chest, head and neck, eye, and prostate. This advanced form of radiation is extremely valuable for the treatment of cancer in pediatric patients, who can be more prone to complications from radiation treatment.
Construction is scheduled to start in the fall, with installation of the Mevion S250 to be complete in 2016.
“The addition of proton therapy to our experienced and internationally known cancer program is a natural progression for us as the only provider in the region with a long history of serving both children as well as adults with cancer,” said Richard Goldberg, M.D., president, MedStar Georgetown University Hospital. “We are excited to bring this important technology to our community and to give our patients yet another cutting-edge cancer treatment option.”
“The Mevion S250 proton therapy system provides a significant benefit to cancer patients while redefining the economics and accessibility of proton therapy,” said Joseph K. Jachinowski, president and CEO of Mevion Medical Systems. “We are proud to bring the most advanced radiation therapy device to our nation’s capital and another leading healthcare institution.”
For more information: www.mevion.com, www.lombardi.georgetown.edu


 January 30, 2026
January 30, 2026 









