December 20, 2013 — Seno Medical Instruments Inc., the company pioneering opto-acoustic imaging as a tool to improve the process of diagnosing breast cancer, announced preliminary information from a feasibility study of its Imagio breast imaging device. Results from the feasibility study suggest that information from Imagio, beyond that available from traditional breast ultrasound, may be helpful in assisting physicians in their decisions whether to recommend biopsies for women with suspicious breast masses. The data were presented at the Radiological Society of America Annual Meeting (RSNA 2013).
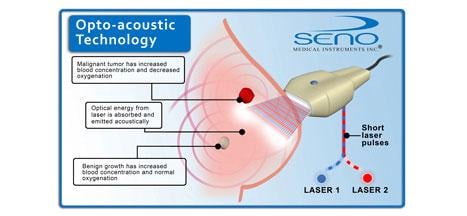
Imagio combines an imaging technology based on light-in and sound-out called "opto-acoustics" with traditional ultrasound. The opto-acoustic images provide a unique blood map in and around suspicious breast masses. Unlike other imaging modalities, Imagio doesn't expose patients to potentially harmful ionizing radiation (X-rays) or injectable contrast agents.
"Given these early results, I believe that opto-acoustics could potentially spare some BI-RADS 4a cases and some BI-RADS 4b cases from needing to undergo biopsies," said Kenneth Kist, M.D., associate professor of Radiology, University of Texas Health Science Center, San Antonio, and study investigator. "This could represent a significant advance in the diagnostic path for breast cancer and I look forward to seeing the results of the ongoing U.S. Pivotal Study."
The BI-RADS classification is used by radiologists to predict the probability of malignancy of a breast mass based on imaging results. A BI-RADS 3 mass only carries up to a 2 percent chance of being cancerous. Patients with masses in the BI-RADS 3 category can safely wait to see if the mass changes or grows. A BI-RADS 4a mass is considered to have more than a 2 percent but less than or equal to 10 percent chance of being cancerous, and a BI-RADS 4b mass is considered to carry from greater than 10 percent to less than or equal to 50 percent risk of malignancy. Patients whose masses are deemed BI-RADS 4a or higher will almost always undergo a core needle or surgical biopsy.
For this study (Abstract LL-BRS-WE5B), five blinded readers independently assessed Imagio images from 73 patients with 74 breast masses. All of the masses were biopsied to provide a gold-standard comparator. Readers were blinded to the subjects' medical history, biopsy report, histology report, clinical records and follow-up. All 34 detected cancer masses remained at their original BI-RADS classification.
In the 40 remaining benign masses, downgrades to BI-RADS 3 were achieved in 12 of 22 original BI-RADS 4a diagnoses as a result of opto-acoustics. Three of 13 BI-RADS 4b masses were downgraded to BI-RADS 3.
Each year in the United States, 1.7 million women undergo core needle or surgical breast biopsies after a suspicious mass is found through breast imaging or self-exams. However, up to four out of five of these biopsies reveal benign pathology.
A separate electronic exhibit (Abstract LL-BRE2487) providing an overview of opto-acoustic technology was also displayed throughout the meeting.
"These early results underscore the potential of opto-acoustic imaging technology which we believe represents a significant step forward in cancer diagnosis," said Thomas Stavros, M.D., FACR, FSRU, FRANZCR, medical director, Seno Medical Instruments. "This feasibility study shows that Imagio has potential to lead to better outcomes for women and fewer biopsies. We are working diligently with 16 leading breast imaging centers across the U.S. to complete our Imagio Pivotal Study that will form the basis of our Premarket Approval Application with the U.S. Food and Drug Administration."
For more information: www.senomedical.com


 February 17, 2026
February 17, 2026 









