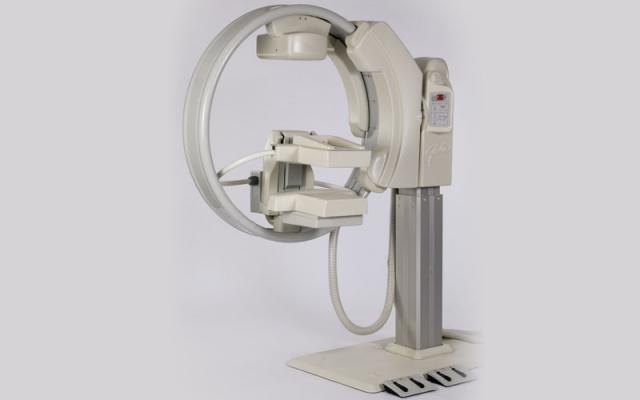
October 29, 2013 — Gamma Medica Inc., developer and marketer of the LumaGem molecular breast
imaging (MBI) system, announced new headquarters and manufacturing facilities in Salem, N.H., supporting expanded commercialization of its flagship LumaGem system and LumaGuide MBI-guided biopsy module.
Last summer, Gamma Medica closed on a $16 million Series A financing round from healthcare investment firm Psilos Group Managers, helping to fund the product design, engineering and manufacturing plant as well as an upcoming expansion of the corporate staff in the new facility.
“This year, we plan to expand product production and launch several additional post-market LumaGem clinical studies,” said Jim Calandra, president and CEO, Gamma Medica. “In addition, Gamma Medica will hire about 40 new employees to support its projected growth.”
LumaGem is a high-resolution imaging system optimized to detect tumors as small as 5mm in
women with difficult-to-image breasts, particularly mammographically dense breasts. Patients whose
mammograms are inconclusive may achieve more precise results with simple, safe and comfortable non-invasive MBI testing at one-third the cost of an MRI.