
The Cios Alpha is a mobile C-arm system designed with greater power output and a larger field of view in the operating room.
January 18, 2013 — At the 98th annual meeting of the Radiological Society of North America (RSNA) in November 2012, Siemens Healthcare demonstrated continued commitment to its Agenda 2013 global initiative by previewing several works-in-progress imaging systems. Systems include cutting-edge technologies in the areas of magnetic resonance imaging (MRI) and radiography. Each system embodies key tenets of Siemens’ Agenda 2013 initiative, a two-year program launched in November 2011 that targets innovation, regional presence, competitiveness and the development of the company’s human resources.
Siemens’ works-in-progress imaging systems include:
- Magnetom Prisma, a 3T MRI system designed with a significantly higher signal-to-noise ratio to tackle demanding clinical and research challenges.
- Cios Alpha, a mobile C-arm system designed with greater power output and a larger field of view in the operating room (OR) than conventional C-arms.
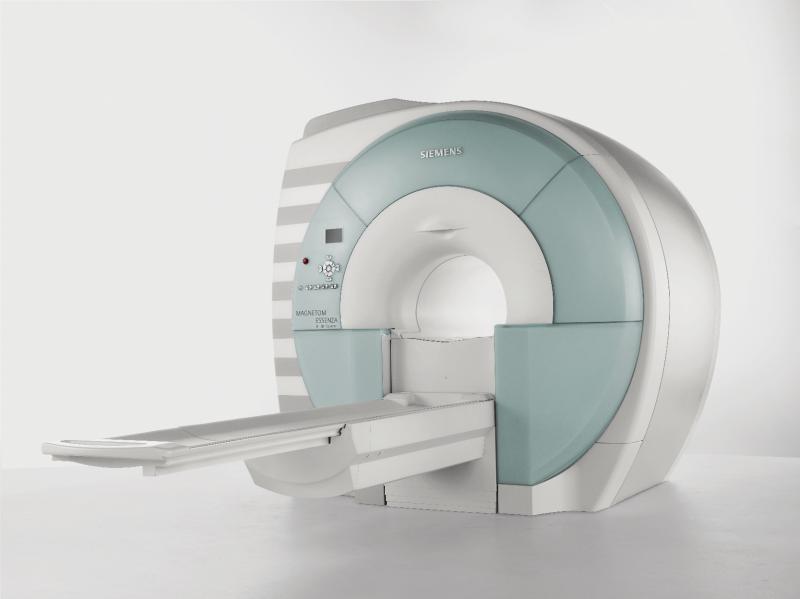
- Magnetom Essenza with Siemens’ MRI workflow solution Dot (Day optimizing throughput), which helps enhance productivity in MR scanning.
- Magnetom Trio, Verio and Avanto upgrades with Dot, which helps enhance productivity in MR scanning, as well as Tim (Total imaging matrix) 4G, the latest generation of Siemens’ integrated coil technology.
- syngo.via WebViewer VA11, which is intended to perform diagnostic reading directly on the iPad as well as provide access to images from computed and digital radiography (CR and DR), positron emission tomography (PET), and PET/computed tomography (CT) devices.[1]
The products mentioned are currently under development and are not for sale in the United States. Their future availability cannot be guaranteed.
For more information: www.siemens.com/healthcare
[1] Under development. Not available for sale in the United States. In the U.S., diagnostic use of syngo.via WebViewer VA11 on mobile devices is not intended to replace a workstation, but only when there is no access to a workstation.



 January 30, 2026
January 30, 2026 









