January 17, 2013 — The PenRad Mammography Information System (MIS) has been named the Category Leader in the “2012 Best in KLAS Awards: Software and Services” report for MIS technology for the fifth consecutive year by KLAS. KLAS rankings are based on product quality, ease-of-use and price, as well as customer support according to evaluations given by system users. Since KLAS began analyzing the MIS market in 2008, PenRad MIS has won the Category Leader each year consecutively.
“Providers recognize the critical nature that vendors play in improving healthcare delivery,” says Adam Gale, KLAS president. “Thus, a growing number of providers are weighing in on vendor performance. It speaks volumes that providers want to be heard and be counted. And vendors are listening.”
“We are extremely proud to again have PenRad MIS recognized as the Category Leader for MIS, a position we have held since the award’s inception in 2008,” said Greg Gustafson, president and CEO of PenRad. “PenRad remains committed to offering innovative products and world class service, ultimately optimizing productivity and revenue in patient image and information management.” Gustafson goes on to say “This achievement means a lot, not only to PenRad but to existing and potential MIS customers as well; a rating of the best in the market is a significant vote of confidence and a differentiator recognized by all.”
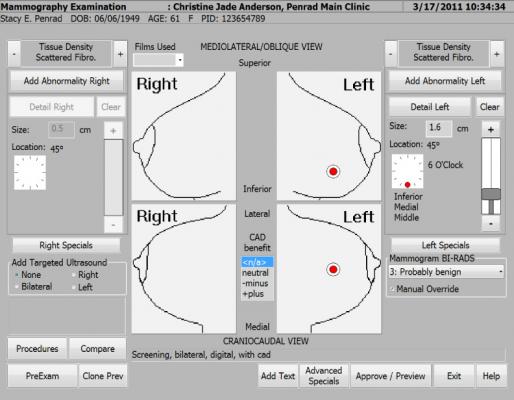
PenRad MIS improves breast imaging workflow by streamlining reporting, tracking and management of clinical data. By automating tasks and eliminating duplication, secondary data input and transcription, PenRad MIS optimizes patient care workflow.
For more information: www.penrad.com


 February 18, 2026
February 18, 2026 









